10 things you need to know today: December 19, 2020
FDA authorizes Moderna's COVID-19 vaccine, Senate passes short term government funding as stimulus negotiations continue, and more


- 1. FDA authorizes Moderna's COVID-19 vaccine
- 2. Senate passes short term government funding as stimulus negotiations continue
- 3. Pompeo: Russia 'pretty clearly' behind cyberattack on U.S.
- 4. Pence, McConnell, Pelosi get COVID-19 vaccine, vouch for its 'safety and efficacy'
- 5. At least 10 killed in attack on political rally in Somalia
- 6. Supreme Court opts against ruling on undocumented immigrants on census
- 7. Abducted Nigerian schoolboys reunited with families
- 8. Stanford Medicine apologizes for vaccine distribution mishap
- 9. Cyberpunk 2077 maker promises refunds after game pulled from PlayStation store
- 10. Conference title games to clarify college football playoff picture
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
1. FDA authorizes Moderna's COVID-19 vaccine
The Food and Drug Administration on Friday authorized Moderna's COVID-19 vaccine for emergency use, making it the second COVID-19 vaccine approved in the U.S. as the country fights record-breaking surges in coronavirus cases and deaths. An FDA advisory panel on Thursday recommended authorizing emergency use of Moderna's coronavirus vaccine, clearing the way for final approval. With emergency use authorization for the vaccine, which was shown in trials to be highly effective in preventing infection, Americans in high-priority groups can begin receiving doses within days. Moderna joins Pfizer's vaccine, which rolled out this week. Millions of doses will be distributed to more than 3,000 locations nationwide; Health care distribution company McKesson said it was ready to immediately ship out dose kits as soon as the FDA approved them.
2. Senate passes short term government funding as stimulus negotiations continue
Senators spent Friday hashing out the final details of a COVID-19 stimulus bill, seeking to pass a package over the weekend. The previous CARES Act expired months ago, and negotiations have been gridlocked ever since. The newfound urgency to pass a deal came before government funding was set to expire at midnight Friday; since negotiations are expected to continue through Monday, lawmakers passed a two-day funding measure late Friday to avoid a short government shutdown. The $900 billion stimulus bill being finalized includes $600 stimulus checks, boosted unemployment benefits of $300 per week, and $325 billion in aid for small businesses. It does not include a corporate liability shield. The latest sticking point is reportedly "over a last-minute Republican effort to cut off the Federal Reserve's ability to restart pandemic relief programs."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
3. Pompeo: Russia 'pretty clearly' behind cyberattack on U.S.
Secretary of State Mike Pompeo said Friday night during an appearance on the Mark Levin Show that "we can say pretty clearly that it was the Russians that engaged" in a recently discovered cyberattack that breached dozens of federal agencies and companies. He added that while "we're still unpacking precisely" what happened, "this was a very significant effort." The New York Times notes Pompeo is the first member of the Trump administration to suggest the Kremlin was behind the attack, even after intelligence agencies have told Congress they suspect Russia's own elite intelligence agency, the S.V.R, was behind it. Russia has denied involvement. President Trump has yet to address the issue, and Pompeo told Levin he may keep quiet during the investigation because sometimes the "wiser course of action to protect the American people is to calmly go about your business and defend freedom."
4. Pence, McConnell, Pelosi get COVID-19 vaccine, vouch for its 'safety and efficacy'
Vice President Mike Pence was inoculated with the Pfizer-BioNTech COVID-19 vaccine on live television Friday morning, which the White House said was to "promote the safety and efficacy of the vaccine and build confidence among the American people." Pence applauded the development of a COVID-19 vaccine in less than a year and said, "while we cut red tape, we cut no corners." Later, House Speaker Nancy Pelosi (D-Calif.) and Senate Majority Leader Mitch McConnell (R-Ky.) also received their vaccines. Pelosi urged Americans to "continue mask wearing, social distancing & other science-based steps to save lives & crush the virus" while the vaccine rolls out. President-elect Joe Biden is set to get the vaccine in a few days and will also do so publicly to vouch for its safety.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
5. At least 10 killed in attack on political rally in Somalia
At least 10 people, including three senior Somali military officers and several local leaders, were reportedly killed Friday in a suicide bombing at a political rally in Galkayo, a town in Somalia's semi-autonomous state of Galmudug. The attack, which was claimed by the al Qaeda-linked Islamist extremist group al Shabab, was aimed at Somalia's Prime Minister Mohamed Hussein Roble, Somali police and al Shabab said. Roble, however, was not harmed in the attack as he was still on his way to the stadium hosting the rally when the explosion took place. In addition to the 10 fatalities, more than 20 people were reportedly injured. The death toll reportedly could continue to rise.
6. Supreme Court opts against ruling on undocumented immigrants on census
The Supreme Court on Friday decided it's too early to challenge President Trump's attempt to discount undocumented immigrants from the census. Over the summer, Trump issued an executive order that would stop undocumented immigrants from counting toward congressional apportionment and federal funding determined by the census. Several states challenged the move in court. In a 6-3 decision, the court said the states' challenge was "not suitable for adjudication at this time." The conservative majority added that it expressed "no view on the merits of the constitutional and related statutory claims presented," merely that it was too early to make a decision. The court's liberal justices disagreed. The decision means Trump can try to remove undocumented people from the apportionment count, and that opposition must sue again later if they'd like to stop the changes from taking effect.
7. Abducted Nigerian schoolboys reunited with families
Shortly after their release was negotiated without violence, more than 300 boys who were kidnapped from their school in northwest Nigeria last week were reunited with their families Friday. The Islamist militant group Boko Haram has claimed responsibility for the kidnapping, but experts are skeptical since it took place outside their usual area of operations. One of the boys said the captors told him to describe them as members of Boko Haram, but he too suspected they were actually unaffiliated armed bandits. During the negotiations the kidnappers raised various grievances about people killing their cattle and various vigilante units disturbing them, further suggesting the incident was related to a longstanding conflict between herders and farming communities in the region.
8. Stanford Medicine apologizes for vaccine distribution mishap
Stanford Medicine apologized Friday for how its COVID-19 vaccine distribution strategy unfolded. The plan, which was apparently based on an algorithm, wound up excluding nearly all medical residents and fellows, many of whom regularly treat coronavirus patients, from the first round of inoculations at the medical center. Only seven of Stanford's 1,300 residents — medical school graduates who staff the hospital while learning specialties — made the cut while medical school faculty and other health-care workers who do not attend to COVID-19 patients received their shots. In an email to staff, Stanford said "we take complete responsibility and profusely apologize to all of you," adding that "we are working quickly to address the flaws in our plan and develop a revised version."
9. Cyberpunk 2077 maker promises refunds after game pulled from PlayStation store
Just over a week after its debut, the highly-anticipated video game Cyberpunk 2077 has already been pulled from Sony's PlayStation Store amid widespread complaints. Sony Interactive Entertainment announced it would be removing the game developed by CD Projekt from the PlayStation Store and offering refunds. Cyberpunk 2077 faced complaints from PlayStation 4 and Xbox One players who encountered frequent glitches. Days after its release, CD Projekt apologized, saying it "should have paid more attention to making it play better on PlayStation 4 and Xbox One." On Friday, it said it would offer refunds "out of our own pocket if necessary." The decision to pull the game from the PlayStation Store entirely "stunned the games market," The Financial Times wrote. CD Projekt said the game was removed "temporarily," adding that it's "working hard" to bring it back "as soon as possible."
10. Conference title games to clarify college football playoff picture
After a tumultuous season affected by the coronavirus pandemic, college football's playoff picture should round into shape Saturday following a slew of conference championship games. No. 4 Ohio State will likely secure a spot in the four-team College Football Playoff with a win against Northwestern in the Big Ten title game at 12 p.m. ET. Later at 4 p.m. ET, No. 3 Clemson and No. 2 Notre Dame, both playoff hopefuls, will square off for the ACC crown in a highly anticipated rematch, and No. 1 Alabama will look to lock up the top seed with a victory over No. 7 Florida in the SEC championship game at 8 p.m. ET. Meanwhile, No. 12 Coastal Carolina and No. 9 Cincinnati, though both playoff longshots, are hoping to cap undefeated seasons with wins in the Sun Belt Conference and American Athletic Conference title games, respectively. On Friday night, Oregon beat USC in the Pac-12 championship game, although the Ducks are not considered a playoff contender.
Tim is a staff writer at The Week and has contributed to Bedford and Bowery and The New York Transatlantic. He is a graduate of Occidental College and NYU's journalism school. Tim enjoys writing about baseball, Europe, and extinct megafauna. He lives in New York City.
-
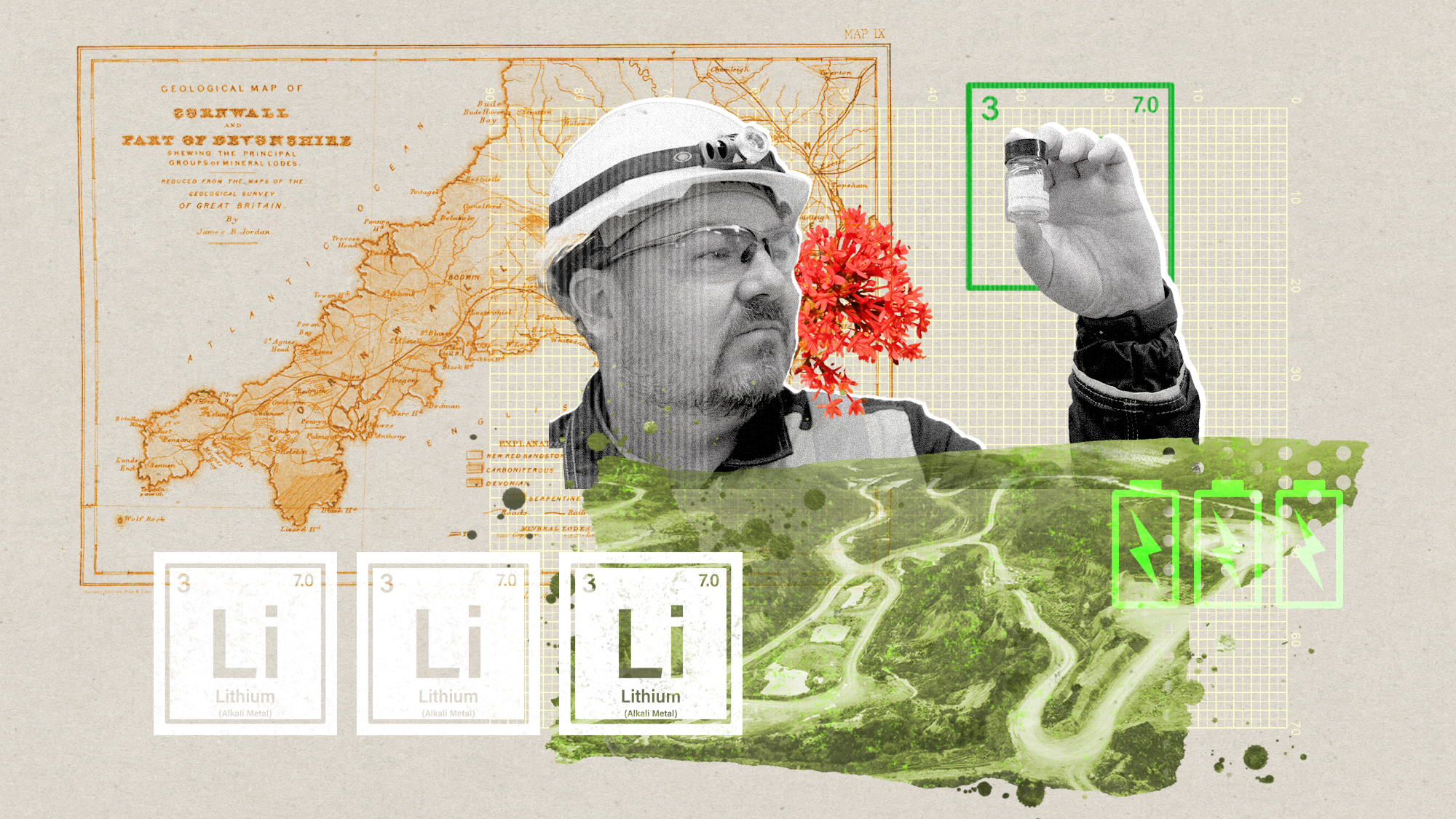 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 10 things you need to know today: January 24, 2024
10 things you need to know today: January 24, 2024Daily Briefing Trump closes in on nomination with New Hampshire win over Haley, 'Oppenheimer' leads the 2024 Oscar nominations, and more
-
 10 things you need to know today: January 23, 2024
10 things you need to know today: January 23, 2024Daily Briefing Haley makes last stand in New Hampshire as Trump extends polling lead, justices side with US over Texas in border fight, and more
-
 10 things you need to know today: January 22, 2024
10 things you need to know today: January 22, 2024Daily Briefing DeSantis ends his presidential campaign and endorses Trump, the US and Arab allies push plan to end Gaza war, and more
-
 10 things you need to know today: January 21, 2024
10 things you need to know today: January 21, 2024Daily Briefing Palestinian death toll reportedly passes 25,000, top Biden adviser to travel to Egypt and Qatar for hostage talks, and more
-
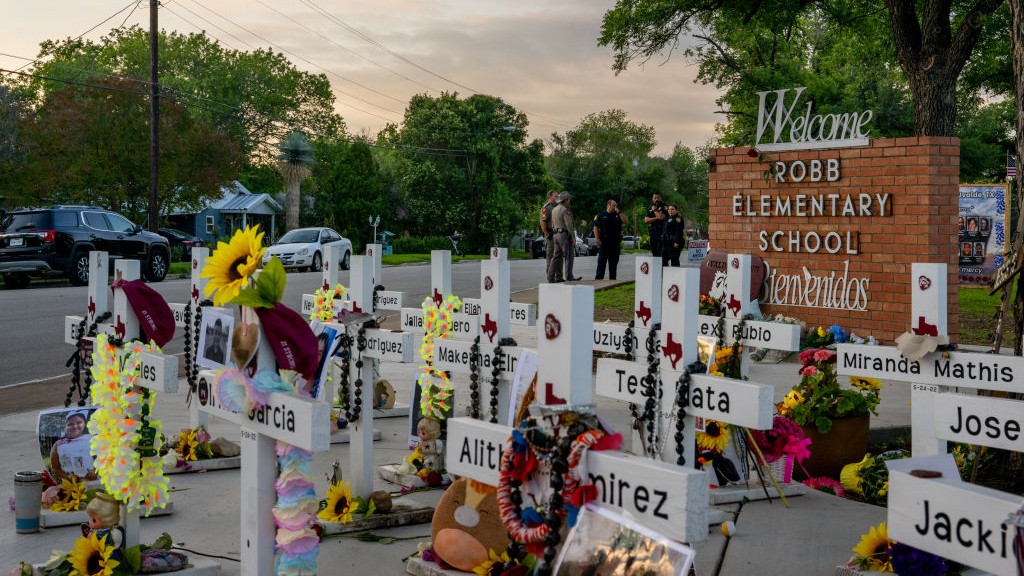 10 things you need to know today: January 20, 2024
10 things you need to know today: January 20, 2024Daily Briefing Grand jury reportedly convened to investigate Uvalde shooting response, families protest outside Netanyahu's house as pressure mounts for hostage deal, and more
-
 10 things you need to know today: January 19, 2024
10 things you need to know today: January 19, 2024Daily Briefing Congress averts a government shutdown, DOJ report cites failures in police response to Texas school shooting, and more
-
 10 things you need to know today: January 18, 2024
10 things you need to know today: January 18, 2024Daily Briefing Judge threatens to remove Trump from his defamation trial, medicine for hostages and Palestinians reach Gaza, and more
-
 10 things you need to know today: January 17, 2024
10 things you need to know today: January 17, 2024Daily Briefing The US strikes Houthi targets in Yemen a third time, Trump's second sex defamation trial begins, and more
