Growing rat kidneys in a lab: Are human organs next?
Doctors make progress toward the ultimate goal of saving patients at risk of dying while awaiting life-saving transplants

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
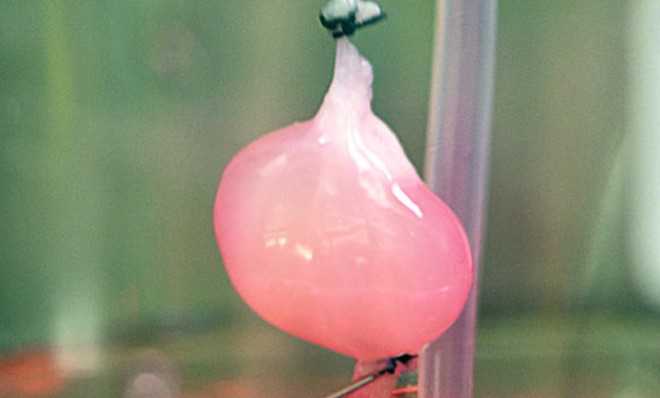
Scientists from Massachusetts General Hospital have used baby-rat cells to grow functioning artificial kidneys in a lab, marking an important step toward the ultimate goal of creating human organs for people awaiting transplants. When implanted into living animals, the bioengineered organs were even able to produce urine. Roughly 100,000 end-stage renal disease patients are waiting in line for donor kidneys in the U.S., and as many as 10,000 die each year before they qualify for a transplant. Is this experiment a sign that doctors are getting close to a breakthrough? Will waiting lists for donor organs become a thing of the past?
The short answer is that there's still far to go. In this experiment, the scientists took a bit of a shortcut. They removed the living cells from a natural rat kidney, leaving them with a frame of collagen. They then filled the hollowed organ again with a mixture of cells, including cells from the kidneys of newborn rats, and wound up with a functioning organ. But there's a catch. "While bioengineered kidneys can produce rudimentary urine, they functioned differently from natural ones," says RT.com, probably because of the immaturity of the renal cells. So the scientists will have to address that problem as they prepare for the next big step.
Before they can realistically hope to use the technique in humans, doctors will try to use a similar process to put one of these lab-grown kidneys into a pig. These animals have an anatomy closer to that of a human's, so such an undertaking would be "a stepping stone to human kidneys," says Sharon Begley at Reuters. The next trials could prove to be a leap from the rat study, as the plan is to use the cellular scaffolding of a pig kidney — similar to what was done in the research with rats — but to repopulate the organ with human cells.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Even once that step is complete, however, the researchers will not be able to promise bio-artificial human kidneys in the near future. "I don't think our place in this organ regeneration effort is to figure it all out, but to show it's a valid platform to get everyone on board — stem cell scientists, transplant surgeons," says Dr. Harald C. Ott, the surgeon and researcher at the Mass. General Center for Regenerative Medicine who led the team that did the study, which was published online on Sunday in the journal Nature Medicine. "My hope is that with this proof of principle, people become aware... and start working on this problem."
Other research groups are working on similar methods to create working organs in the lab. Competing labs are using 3D bioprinters to create the framework for the new organs. "With a 3D bioprinter, you wouldn't require donor organs," said Dr. Anthony Atala, a pioneer in the technology at the Institute for Regenerative Medicine at Wake Forest School of Medicine in North Carolina. "The printer also lets you be very precise in where the cells go." Ultimately, all research in the field is focused on the same goal — saving, potentially, thousands of lives a year by allowing people to get new organs without languishing on donor lists, and creating their new kidneys, livers, even hearts, with their own cells to get around the danger of organ rejection. Doctors have already managed to replace more simple body parts — surgeons in Spain replaced a woman's collapsed windpipe with one created in a lab. Major organs could very well be next.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Harold Maass is a contributing editor at The Week. He has been writing for The Week since the 2001 debut of the U.S. print edition and served as editor of TheWeek.com when it launched in 2008. Harold started his career as a newspaper reporter in South Florida and Haiti. He has previously worked for a variety of news outlets, including The Miami Herald, ABC News and Fox News, and for several years wrote a daily roundup of financial news for The Week and Yahoo Finance.
-
 Crisis in Cuba: a ‘golden opportunity’ for Washington?
Crisis in Cuba: a ‘golden opportunity’ for Washington?Talking Point The Trump administration is applying the pressure, and with Latin America swinging to the right, Havana is becoming more ‘politically isolated’
-
 5 thoroughly redacted cartoons about Pam Bondi protecting predators
5 thoroughly redacted cartoons about Pam Bondi protecting predatorsCartoons Artists take on the real victim, types of protection, and more
-
 Palestine Action and the trouble with defining terrorism
Palestine Action and the trouble with defining terrorismIn the Spotlight The issues with proscribing the group ‘became apparent as soon as the police began putting it into practice’
