Polypill: The magic pill that could add 11 years to your life
A new four-in-one cocktail of cardiovascular medications could help prevent heart attacks and stroke in adults over the age of 50
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
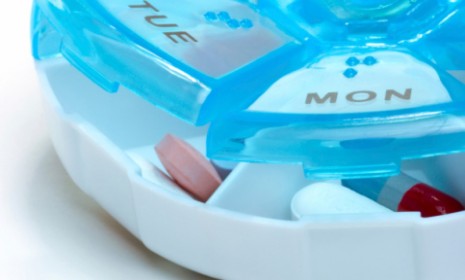
A new multipurpose pill that reduces blood pressure and cholesterol while lowering a person's risk of heart attack and stroke could ostensibly save thousands of lives a year, according to a new British study. The "polypill" is intended to make life remarkably easier for adults over age 50, and some doctors predict it could even be a game changer. Here's what you need to know:
How does it work?
The "four-in-one pill," which is meant to be taken every night, combines three generic blood pressure medicines and a cholesterol fighting drug called simavastatin. The idea of a "one-size-fits-all" cocktail of heart drugs has been floating around for roughly a decade, says Ben Hirschler at Reuters, "but its path to market remains unclear given regulatory hurdles" coupled with "a lack of interest from big drugmakers focused on selling new patented medicines."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Then why make it?
Roughly 200,000 people die annually of cardiovascular complications in the U.K., says Stephen Adams at the Telegraph. If adults over 50 were prescribed this pill, that number could be cut by a little more than half, according to scientists from Queen Mary University of London.
How did researchers learn that?
In a small study of 84 adults, the polypill cut blood pressure by 12 percent and lowered so-called "bad" cholesterol by 39 percent. Consequently, the team predicts that taking it could cut heart disease events by 72 percent and stroke by 64 percent. "But not all would benefit," says the Telegraph's Adams: "Some who take the pill would still suffer a heart attack or stroke, while for others such an event would be delayed." On average, patients on the pill could gain an extra 11 years of life.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Are there any side effects?
A few patients during the three-month study said they experienced muscle aches due to the statin in the polypill, but that wasn't enough for them to want to discontinue treatment.
So what are the next steps for the polypill?
Before it can be sold, it'll have to make it past safety regulators, a process that could take one to two years. Larger trials in India are also underway. "We need trial evidence that looks at real life outcomes like death rates," says Dr. Maraget McCartney, who wasn't involved with the study. "We need much larger trials that give us fair representations of risk and harm."
Sources: CBS News, Med Page Today, Reuters, Telegraph
-
 Health insurance: Premiums soar as ACA subsidies end
Health insurance: Premiums soar as ACA subsidies endFeature 1.4 million people have dropped coverage
-
 Anthropic: AI triggers the ‘SaaSpocalypse’
Anthropic: AI triggers the ‘SaaSpocalypse’Feature A grim reaper for software services?
-
 NIH director Bhattacharya tapped as acting CDC head
NIH director Bhattacharya tapped as acting CDC headSpeed Read Jay Bhattacharya, a critic of the CDC’s Covid-19 response, will now lead the Centers for Disease Control and Prevention