The 'smart bomb' therapy that blasts breast cancer
A promising new study shows that a powerful drug can attach directly to tumor cells while leaving healthy cells relatively untouched
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
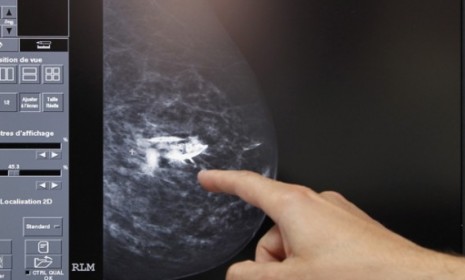
Researchers are excited about a new cancer therapy that explicitly targets breast cancer cells while leaving healthy ones alone. The experimental "smart bomb," which attaches directly to tumors before killing them, is being hailed as "a major step forward" in the fight against cancer and could potentially reach the market within a year. Here's what you should know about the breakthrough:
Why is it called a "smart bomb"?
The treatment, referred to by doctors as T-DM1, combines two proven therapies to blast cancer cells: The drug Herceptin, a gene-targeted therapy sometimes used on patients whose tumors overproduce a specific protein, and a cancer-blasting form of chemotherapy so poisonous that it can't be administered alone. The two are bonded together by a chemical that unleashes when it comes into contact with breast cancer cells, delivering a potent payload that — unlike other cancer-fighting treatments — leaves healthy cells out of harm's way. Hence, T-DM1's "smart bomb" moniker.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
How effective is it?
About 1,000 women with advanced breast cancer were given the drug. After two years, 65 percent of the women who received T-DM1 were still alive, versus 47 percent of a control group that was given standard treatment. More tests are necessary, however, because the margin was just below the rigid criteria cancer researchers use before declaring a new treatment a winner.
Why is this such a breakthrough?
It's the first study to prove that tumors can be precisely targeted with an antibody, minimizing uncomfortable side effects. "People don't lose their hair, they don't throw up. They don't need nausea medicines, they don't need transfusions," study leader Dr. Kimberly Blackwell of Duke University tells The Associated Press.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
When might T-DM1 become available?
The drug's parent company, Roche, plans to file for FDA marketing approval in the U.S. and Europe by the end of the year. Shanu Modi, a New York breast cancer oncologist, who was not involved in the study, tells Bloomberg Businessweek that if T-DM1 is approved, "it is going to be rapidly taken up by the oncology community. For sure, I will be using a lot of it."
Sources: Associated Press, Bloomberg Businessweek, Wall Street Journal
-
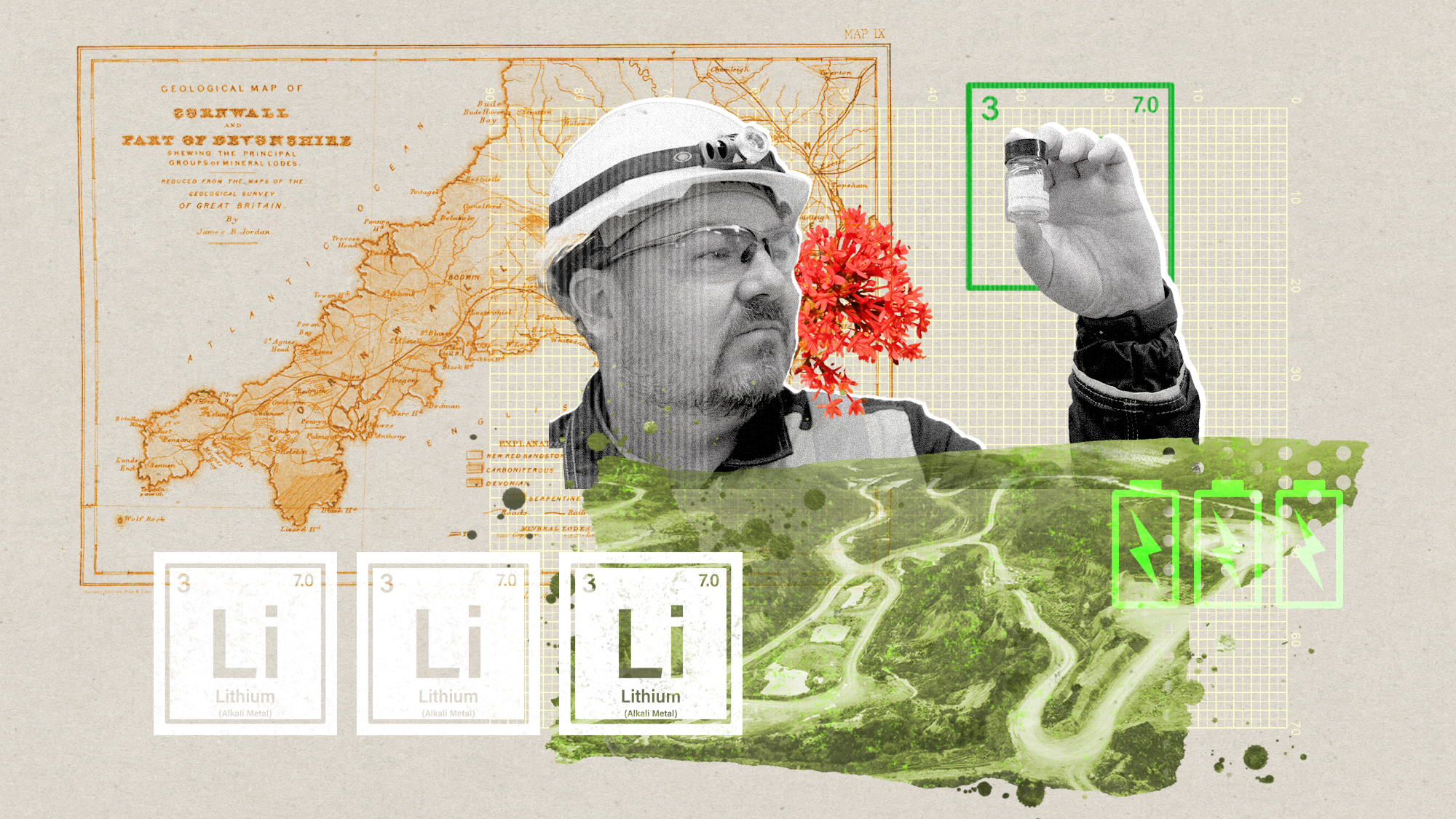 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day