The U.S. could double its COVID-19 vaccine availability overnight. What's the holdup?
How the FDA could approve a more efficient vaccine rollout

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
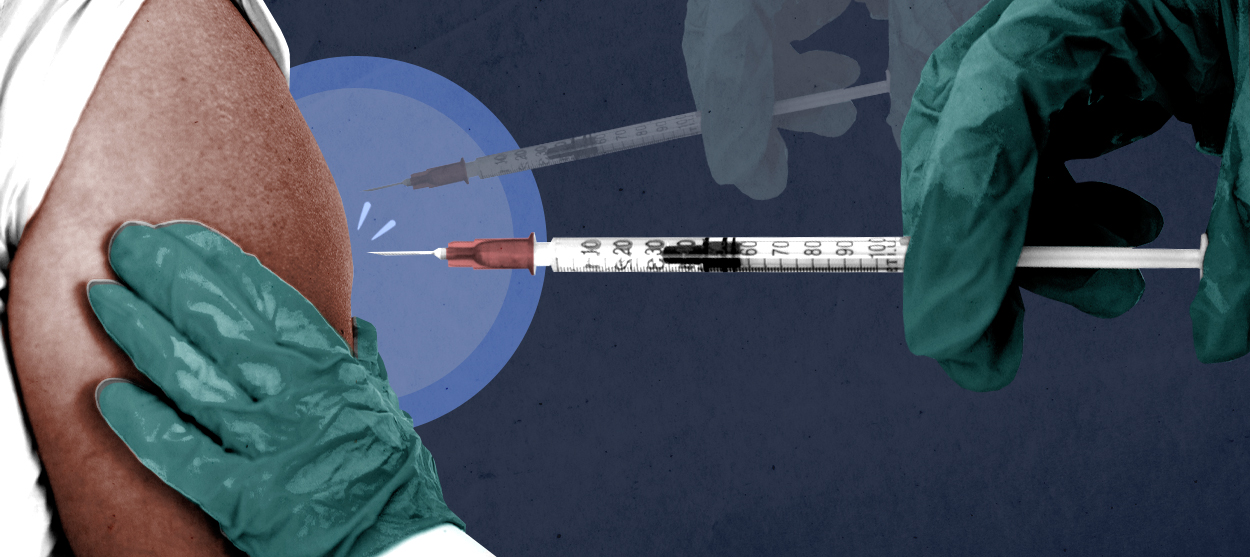
What if we could instantly double COVID-19 vaccine availability in America?
This is the tantalizing prospect raised by data collected while testing the double-dose regimen for the Moderna and Pfizer vaccines. As two Canadian researchers highlighted in a letter to the New England Journal of Medicine this month, both vaccines have been found to achieve 92 percent efficacy 14 days after a recipient has been given just one dose. The second dose, administered three to four weeks after the first, offers comparatively small gains by this measure: It boosts the Pfizer vaccine's efficacy to 95 percent, and the Moderna vaccine's to 94 percent, differences of just three and two points, respectively.
"With such a highly protective first dose, the benefits derived from a scarce supply of vaccine could be maximized by deferring second doses until all priority group members are offered at least one dose," the letter argues. In other words, it's possible we could more efficiently slow the viral spread and prevent thousands of deaths and hospitalizations in America if we saved the second shot for later, when supplies are larger.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Could a single-dose plan actually happen here in the United States? Would the FDA ever permit it, especially in the relatively short time span where this shift could make a big difference? Or is delaying the second dose a pipe dream?
The Canadian researchers dubbed such a strategic shift "a matter of national security" — strong words, and not unwarranted. Vaccine availability has increased, but present forecasts don't see us hitting the target of 3 million per day until April. The current average is just 1.4 million per day, and of the 267 million people eligible for COVID-19 vaccination in America (the vaccines aren't approved for children yet), just 7 percent are fully vaccinated and 14 percent have one shot. We're going too slow.
I reached out to I. Glenn Cohen, a professor of health law and bioethics at Harvard University, and Holly Fernandez Lynch, an assistant professor of medical ethics at the University of Pennsylvania. Together they edited FDA in the Twenty-First Century: The Challenges of Regulating Drugs and New Technologies, which explores the FDA's function, successes, and failures.
Both experts told me the move to a one-dose regimen here in the U.S. would be possible — at least theoretically. "If there are adequate data to support moving to a single dose of any of the two-dose vaccines, I would expect [the FDA] to amend the emergency use authorizations accordingly," Fernandez Lynch told me in an email. But, she said, there's a catch: "They can only act on applications submitted to the agency." That means the ball is in Moderna and Pfizer's court. They would have to call for the FDA to change its use authorization for their products to allow for delaying the second dose.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
If the companies do apply for a change, Cohen said he would expect the FDA to move quickly enough to give an answer within the critical period of vaccine shortages this spring. "As agencies go, FDA is appropriately careful in their review of these matters," he said, "but given the amount of data experience they have with Pfizer and Moderna thus far, if provided appropriate data, I think they could probably make a determination of whether such a change was warranted fairly quickly."
But that answer wouldn't necessarily be "yes." Cohen explained that while many people in the public health sphere have lobbied for the change, the powers that be are somewhat hesitant. He pointed to a recent Meet the Press appearance by National Institute of Allergy and Infectious Diseases Director Anthony Fauci, during which he signaled the FDA wasn't keen on the idea of a single dose regimen. Fauci raised concerns about immunity durability (how long the vaccine's protection against COVID-19 will last) and noted that the second dose increases the number of antibodies significantly.
The durability issue is a legitimate uncertainty. Wilson, the Yale professor, acknowledged that concern but concluded a single shot is worth trying anyway. The vaccine makers may not come to the same conclusion. Thus far they've been quite cautious about the idea, because they haven't yet conducted their own trials measuring single-dose efficacy. A Pfizer representative reiterated as much to me in an email, explaining that the company doesn't "have any Pfizer-led analysis on a single-dose program, so our guidance is to follow the current label under the FDA's [Emergency Use Authorization]." The email added that the Pfizer "vaccine showed a 52 percent vaccine efficacy after the first dose and then more than 95 percent after two." (I also reached out to Moderna, but did not receive a reply.)
But that much less impressive 52 percent figure Pfizer mentioned comes from measurements taken before the 14-day mark after administration, while the immune response is still developing. The data the Canadian researchers touted shows it hits 92 percent at the end of those two weeks, when the body has had more time to react. Plus, as Reason reported in consideration of this idea in December, far lower efficacy, including in the 50 percent range, is routinely considered adequate for pneumonia and seasonal flu vaccines.
As Wilson said, "the primary problem people have with this analysis" recommending a single dose is not that the numbers aren't impressive; it's "that this isn't what the study was designed to do." He decided the risk is low enough and the pandemic deadly enough to try a single dose even given the unusual way that 92 percent number was obtained.
For some idea on how a single-dose strategy would play out, look to the United Kingdom, which has been delaying the second shot of the vaccines from both Pfizer and AstraZeneca, another vaccine maker. The decision, which was made by a U.K. health regulatory agency, was seen as both controversial and risky. Pfizer protested, saying its Phase 3 vaccine study was only meant to "evaluate the vaccine's safety and efficacy following a 2-dose schedule, separated by 21 days. The safety and efficacy of the vaccine has not been evaluated on different dosing schedules."
To borrow Fernandez Lynch's phrase from our conversation, moving to one shot is "a gamble." The U.K. did it anyway, in a desperate attempt to curtail its huge third wave of infections brought on by a more contagious new variant. And the gamble seems to have paid off. Early data from health-care workers who got the Pfizer vaccine shows "clear protection from the first dose, particularly against severe disease." Nearly 30 percent of the U.K. population now has some level of immunity from vaccination, and cases are in decline. In the U.S., by contrast, cases are again on the uptick.
As AstraZeneca researchers have said, giving the second dose three months after the first "is an effective strategy for reducing disease, and may be the optimal for rollout of a pandemic vaccine when supplies are limited in the short term." And for now, supplies in America remain very limited. But they don't have to be. The U.S. should consider the one-shot plan. It seems that's not impossible — but the vaccine makers have to ask for it.
Bonnie Kristian was a deputy editor and acting editor-in-chief of TheWeek.com. She is a columnist at Christianity Today and author of Untrustworthy: The Knowledge Crisis Breaking Our Brains, Polluting Our Politics, and Corrupting Christian Community (forthcoming 2022) and A Flexible Faith: Rethinking What It Means to Follow Jesus Today (2018). Her writing has also appeared at Time Magazine, CNN, USA Today, Newsweek, the Los Angeles Times, and The American Conservative, among other outlets.
-
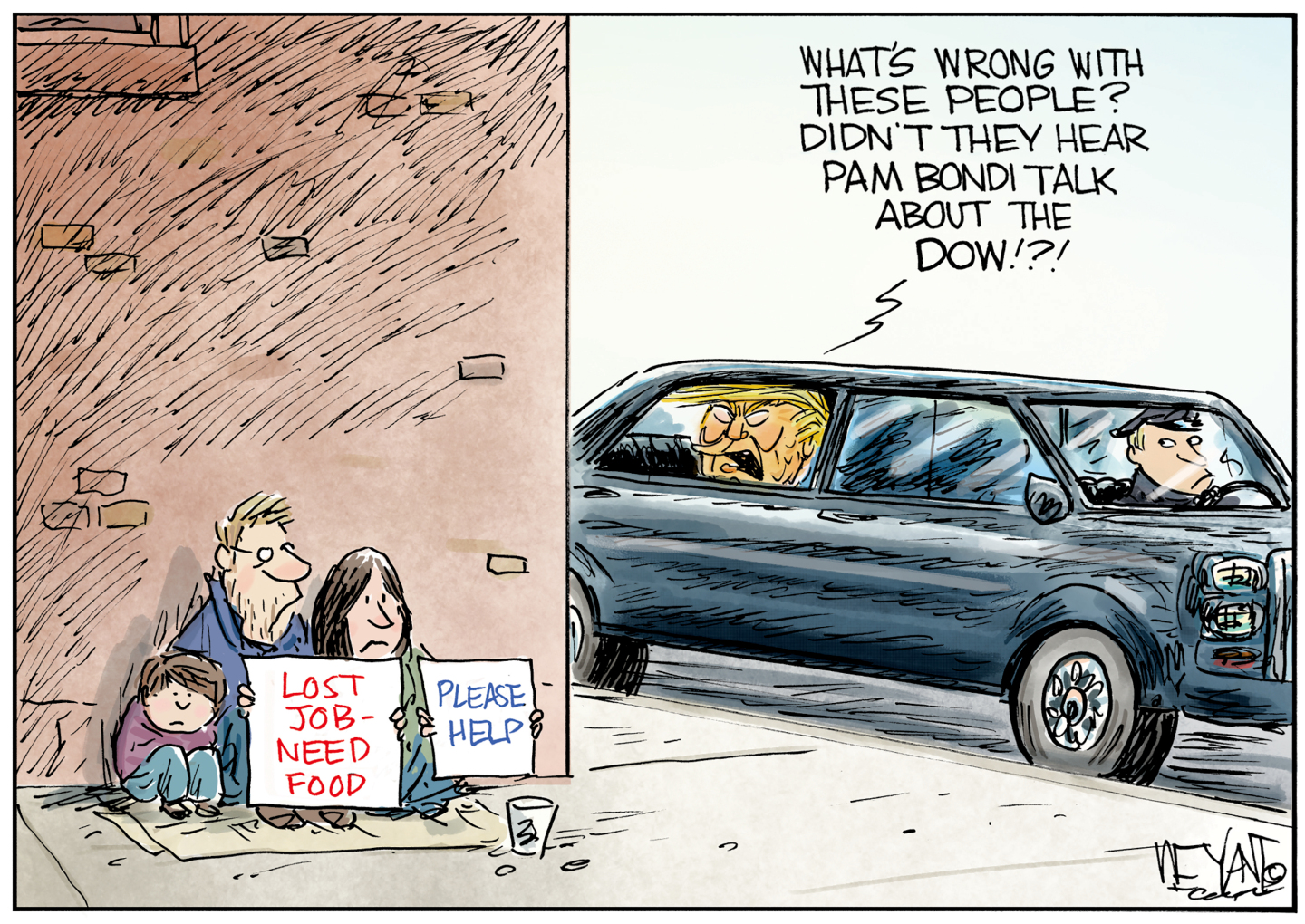 Political cartoons for February 18
Political cartoons for February 18Cartoons Wednesday’s political cartoons include the DOW, human replacement, and more
-
 The best music tours to book in 2026
The best music tours to book in 2026The Week Recommends Must-see live shows to catch this year from Lily Allen to Florence + The Machine
-
 Gisèle Pelicot’s ‘extraordinarily courageous’ memoir is a ‘compelling’ read
Gisèle Pelicot’s ‘extraordinarily courageous’ memoir is a ‘compelling’ readIn the Spotlight A Hymn to Life is a ‘riveting’ account of Pelicot’s ordeal and a ‘rousing feminist manifesto’
-
 Do unvaccinated COVID patients deserve scarce care? A doctor weighs in.
Do unvaccinated COVID patients deserve scarce care? A doctor weighs in.The Explainer Justice, judgment, and the last ICU bed
-
 How to vaccinate the anti-vaxxers
How to vaccinate the anti-vaxxersThe Explainer Instead of blaming people for not doing the right thing, let's focus on eliminating the obstacles to vaccination that still remain
-
 The October Surprise nobody wanted
The October Surprise nobody wantedThe Explainer Trump has COVID-19. Really, 2020?
-
 Life is worth living
Life is worth livingThe Explainer What's driving America's rising suicide rate?
-
 Social workers are masters at de-escalation. Here's what the police can learn from them.
Social workers are masters at de-escalation. Here's what the police can learn from them.The Explainer Knowing how to peacefully resolve conflict, rather than exacerbate it, can save lives
-
 Settling in for the long pandemic
Settling in for the long pandemicThe Explainer Life won't be back to "normal" anytime soon
-
 Sports reveal how much America is trailing the rest of the world
Sports reveal how much America is trailing the rest of the worldThe Explainer MLS and other American leagues are stumbling through their pandemic restart plans
-
 Nobody knows how bad this coronavirus surge is
Nobody knows how bad this coronavirus surge isThe Explainer To make good policy, we need to know what's actually happening
