The daily business briefing: May 18, 2022
FDA authorizes 1st COVID booster for kids ages 5 to 11, Target shares drop as high costs hurt profit, and more

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
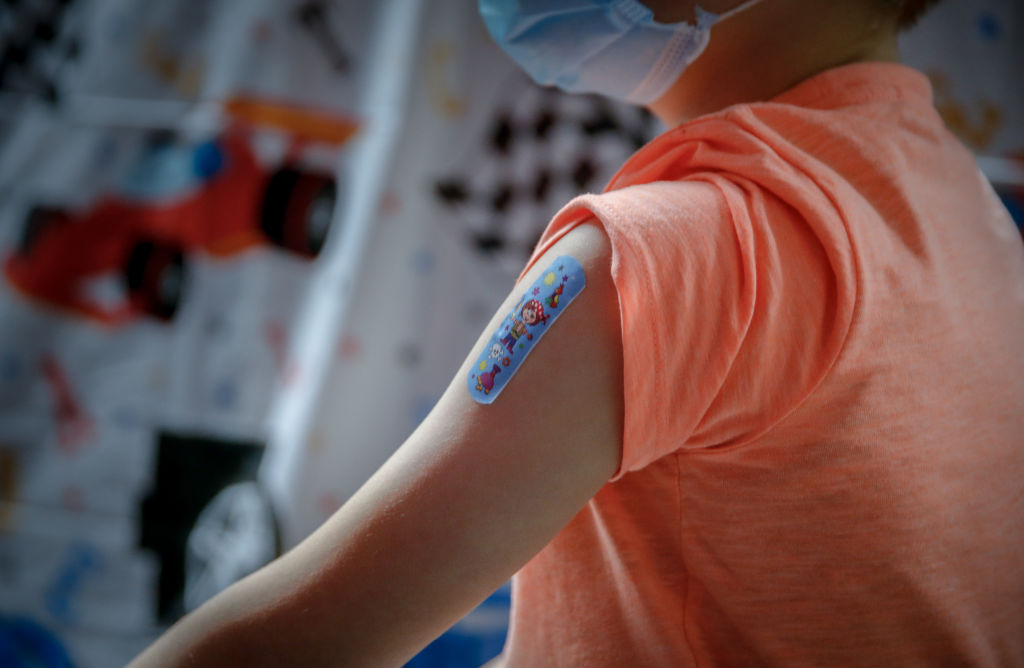
1. FDA authorizes Pfizer booster for kids ages 5 to 11
The Food and Drug Administration on Tuesday authorized giving the Pfizer-BioNTech COVID-19 vaccine booster to children ages 5 to 11. Children in that age group will be eligible for the third dose of the vaccine five months after they received the second shot. The companies asked the FDA to approve the booster, the first available to children that young, based on a small study they said indicated it was safe and effective in boosting antibody levels to counter waning immunity. Previously, only people 12 or older were able to get booster shots. The companies said the booster increased protection against the coronavirus, including the highly infectious Omicron variant, which has increased the number of children hospitalized with COVID-19.
2. Target shares fall as high costs dent profit
Target shares plunged by 16 percent in pre-market trading on Wednesday after the retailer reported disappointing quarterly profits due to rising freight and inventory costs. Target reported strong quarterly net sales of $25.17 billion, beating the $24.47 billion analysts expected, but the cost increases caused diluted earnings per share to come in at $2.19, far short of the $3.07 expected. "We never expected the kind of cost increases in freight and transportation that we're seeing right now," Target Chairman and CEO Brian Cornell told Yahoo Finance. Target expects another $1 billion in freight and transportation costs this year related to high fuel and diesel prices. Like Walmart, it slashed its profit outlook for the year after the rough start.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
3. U.S. lets Chevron talk to Venezuela about resuming oil sales
The Treasury Department on Tuesday gave Chevron a "narrow" license that will allow the energy giant to start talks with Venezuela to resume oil production halted under U.S. sanctions against the South American country's socialist government, The Washington Post reported, citing U.S. officials. The license marked the first step toward possible sanctions relief under discussion since Russia's invasion of Ukraine triggered new sanctions on Russia and disrupted global oil markets. Successful talks could clear Chevron to extract and sell Venezuelan crude. Renewing purchases of Venezuelan oil could increase available supply while driving a wedge between the government of controversial Venezuelan President Nicolas Maduro and Russia, a close ally.
4. Powell says Fed will curb inflation even if 'some pain involved'
Federal Reserve Chair Jerome Powell told The Wall Street Journal in a Tuesday interview that the central bank is committed to bringing down the highest inflation in 40 years, even if there is "some pain involved." "Restoring price stability is an unconditional need. It is something we have to do," Powell said during the Journal's Future of Everything Festival. Powell said he hoped the Fed's efforts, which include interest rate hikes and a reduction of its balance sheet, will do the trick without weakening the labor market, but that bringing down price pressures could result in a slight rise in unemployment, which was near a half-century low at 3.6 percent in April.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
5. Stock futures fall slightly after Tuesday rally
U.S. stock futures fell slightly early Wednesday following Tuesday's rally. Futures tied to the Dow Jones Industrial Average and the S&P 500 were down 0.3 percent and 0.4 percent at 7 a.m. ET. Nasdaq futures were down 0.7 percent. On Tuesday, the Dow and the S&P gained 1.3 percent and 2 percent, respectively, and the tech-heavy Nasdaq jumped 2.8 percent, after a recent sell-off blamed on numerous factors, including concerns about inflation, rising interest rates, and Russia's invasion of Ukraine. The Dow has fallen for seven straight weeks, and the S&P 500 fell close to bear-market territory, defined as 20 percent below its record high, but has risen 4 percent since Thursday.
Harold Maass is a contributing editor at The Week. He has been writing for The Week since the 2001 debut of the U.S. print edition and served as editor of TheWeek.com when it launched in 2008. Harold started his career as a newspaper reporter in South Florida and Haiti. He has previously worked for a variety of news outlets, including The Miami Herald, ABC News and Fox News, and for several years wrote a daily roundup of financial news for The Week and Yahoo Finance.
-
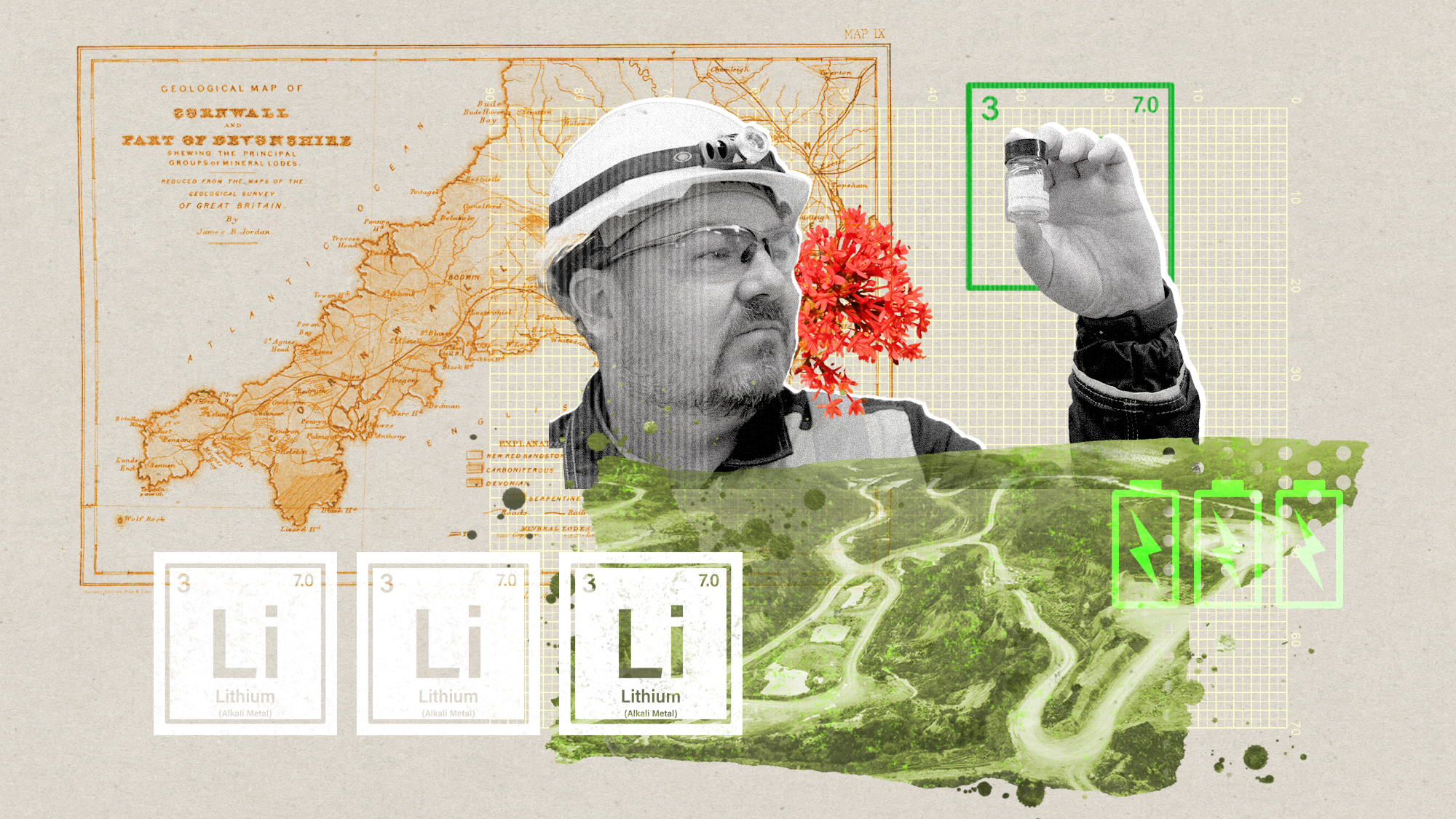 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
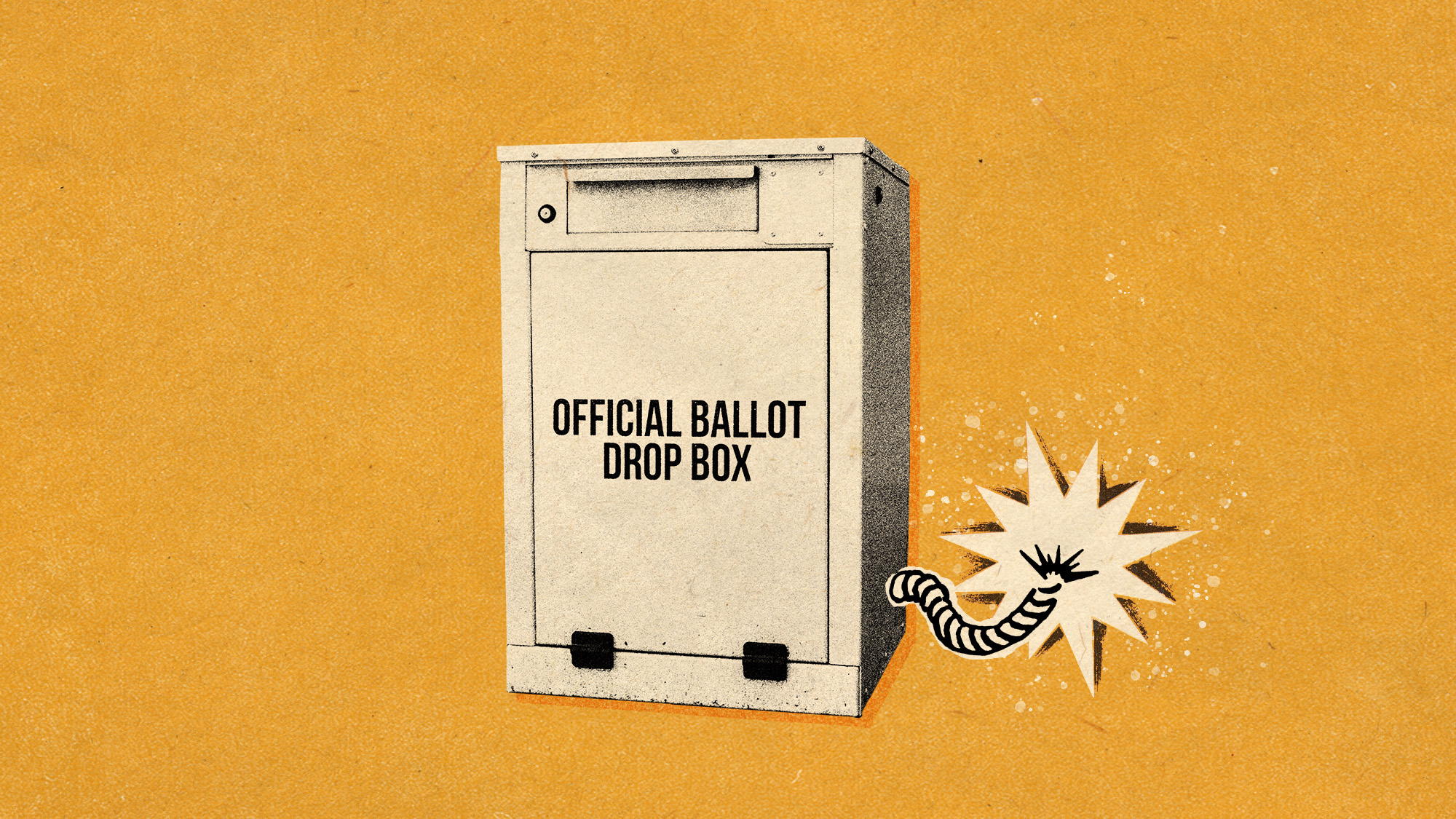 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 The daily business briefing: January 24, 2024
The daily business briefing: January 24, 2024Business Briefing The S&P 500 sets a third straight record, Netflix adds more subscribers than expected, and more
-
 The daily business briefing: January 23, 2024
The daily business briefing: January 23, 2024Business Briefing The Dow and S&P 500 set fresh records, Bitcoin falls as ETF enthusiasm fades, and more
-
 The daily business briefing: January 22, 2024
The daily business briefing: January 22, 2024Business Briefing FAA recommends inspections of a second Boeing 737 model, Macy's rejects Arkhouse bid, and more
-
 The daily business briefing: January 19, 2024
The daily business briefing: January 19, 2024Business Briefing Macy's to cut 2,350 jobs, Congress averts a government shutdown, and more
-
 The daily business briefing: January 18, 2024
The daily business briefing: January 18, 2024Business Briefing Shell suspends shipments in the Red Sea, December retail sales beat expectations, and more
-
 The daily business briefing: January 17, 2024
The daily business briefing: January 17, 2024Business Briefing Judge blocks JetBlue-Spirit merger plan, Goldman Sachs beats expectations with wealth-management boost, and more
-
 The daily business briefing: January 16, 2024
The daily business briefing: January 16, 2024Business Briefing Boeing steps up inspections on 737 Max 9 jets, Zelenskyy fights for world leaders' attention at Davos, and more
-
 The daily business briefing: January 12, 2024
The daily business briefing: January 12, 2024Business Briefing Inflation was slightly hotter than expected in December, Hertz is selling a third of its EVs to buy more gas cars, and more
