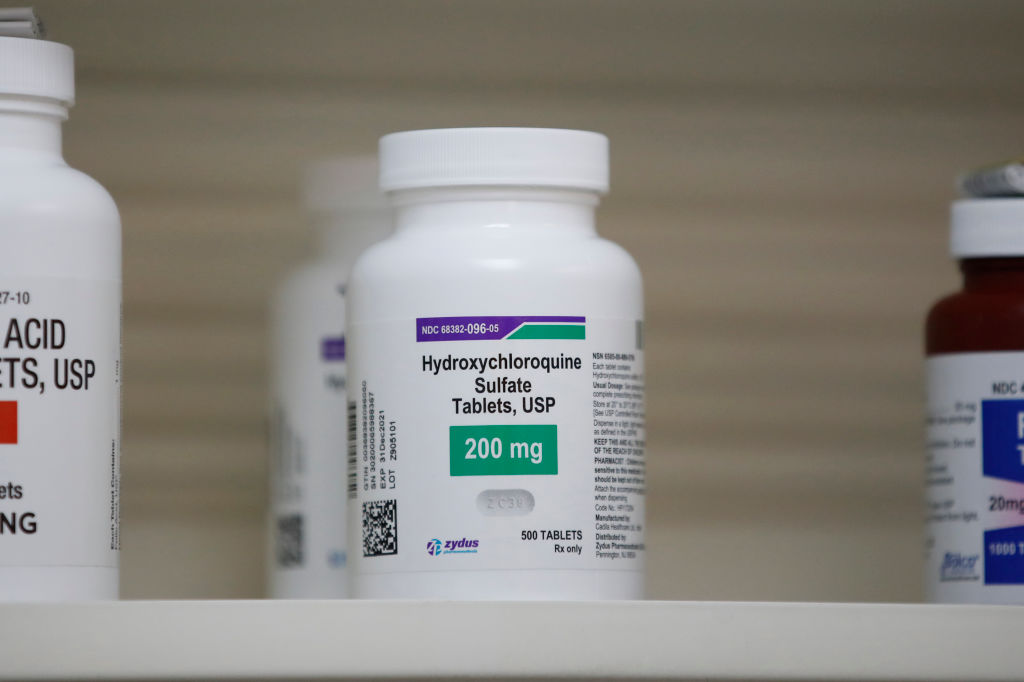
FDA withdraws coronavirus emergency use authorization for hydroxychloroquine

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
At the request of Gary Disbrow, the acting director of the Biomedical Advanced Research and Development Authority, the Food and Drug Administration on Monday withdrew emergency use authorizations for hydroxychloroquine and chloroquine related to coronavirus treatment.
The authorizations were controversial, as many skeptics believed they were made because President Trump had touted the malaria drugs as effective treatments against COVID-19, despite researchers concerns about potential heart-related side effects. Ultimately, after reviewing new information from large clinical trials, the FDA said it does not believe the drugs are likely "to produce an antiviral effect" against the novel virus.
Hydroxychloroquine is approved for several uses like treating arthritis and lupus, so doctors could still use it "off label" to treat coronavirus patients, and clinical trials examining its effect against COVID-19 can continue, Politico reports. The version of chloroquine temporarily authorized by the FDA, however, is not approved in the U.S., so all use of it will end. Read more at Politico.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Tim is a staff writer at The Week and has contributed to Bedford and Bowery and The New York Transatlantic. He is a graduate of Occidental College and NYU's journalism school. Tim enjoys writing about baseball, Europe, and extinct megafauna. He lives in New York City.
-
 6 of the world’s most accessible destinations
6 of the world’s most accessible destinationsThe Week Recommends Experience all of Berlin, Singapore and Sydney
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 What is the endgame in the DHS shutdown?
What is the endgame in the DHS shutdown?Today’s Big Question Democrats want to rein in ICE’s immigration crackdown
-
 Nobody seems surprised Wagner's Prigozhin died under suspicious circumstances
Nobody seems surprised Wagner's Prigozhin died under suspicious circumstancesSpeed Read
-
 Western mountain climbers allegedly left Pakistani porter to die on K2
Western mountain climbers allegedly left Pakistani porter to die on K2Speed Read
-
 'Circular saw blades' divide controversial Rio Grande buoys installed by Texas governor
'Circular saw blades' divide controversial Rio Grande buoys installed by Texas governorSpeed Read
-
 Los Angeles city workers stage 1-day walkout over labor conditions
Los Angeles city workers stage 1-day walkout over labor conditionsSpeed Read
-
 Mega Millions jackpot climbs to an estimated $1.55 billion
Mega Millions jackpot climbs to an estimated $1.55 billionSpeed Read
-
 Bangladesh dealing with worst dengue fever outbreak on record
Bangladesh dealing with worst dengue fever outbreak on recordSpeed Read
-
 Glacial outburst flooding in Juneau destroys homes
Glacial outburst flooding in Juneau destroys homesSpeed Read
-
 Scotland seeking 'monster hunters' to search for fabled Loch Ness creature
Scotland seeking 'monster hunters' to search for fabled Loch Ness creatureSpeed Read
