'Female Viagra': why the libido-boosting drug is so controversial
Flibanserin has finally been approved by the US drugs agency – but only after two unsuccessful attempts
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
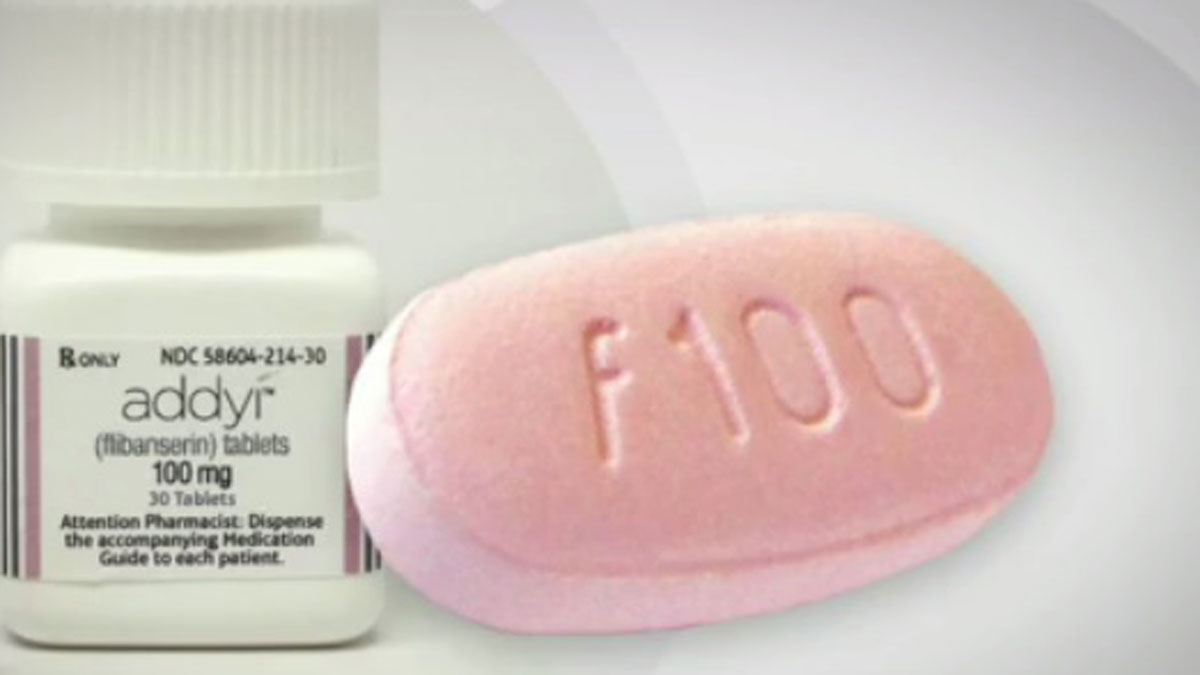
The first drug to treat a lack of sexual desire in women has been approved by the US Food and Drug Administration, but it continues to divide medical professionals.
"Today's approval provides women distressed by their low sexual desire with an approved treatment option," said Janet Woodcock, M.D., director of the FDA’s Center for Drug Evaluation and Research.
But Flibanserin has already been rejected twice by the drugs agency and there are widespread concerns about the safety and efficacy of the much-anticipated pill nicknamed the "female Viagra".
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
What is it and how does it work?
The drug, sold under the brand name Addyi and produced by Sprout Pharmaceuticals, is designed to treat low libido in premenopausal women. One small pink pill is taken daily by women who have been diagnosed with Hypoactive Sexual Desire Disorder. Unlike Viagra, the drug does not relax muscles or increase blood flow, but changes brain chemistry similar to the way that antidepressants do, The Guardian reports.
The support
Dr Lauren Streicher of Northwestern University told NBC News that the FDA approval is a "huge advancement" in women's health. "As a sexual health expert, as a gynaecologist, as someone who takes care of women every single day, this is going to be a game changer for me because right now I have women that come into my office and have a distressing lack of sexual desire and I just say, 'I'm so sorry. There is nothing that I can do.'"
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
The controversy
But critics argue that the drug is simply not effective enough. According to FDA analysis, only between 8 and 13 percent of women who take the pill will see some improvement. "That's a pretty small number, particularly when you consider how "improvement" has been defined," says Vox. Women taking the pill have on average just 0.5 more "sexually satisfying events" per month.
Dr. Adriane Fugh-Berman from Georgetown University has described the drug as "a mediocre aphrodisiac with scary side effects". These include very low blood pressure, dizziness, sleepiness and even loss of consciousness. Drinking alcohol and taking some forms of birth control will increase the risk of the side-effects.
Evidence of the drug's safety and efficacy has not improved since it was last rejected, and many have suggested that the FDA approval was a result of a sustained lobbying campaign by drug makers. Sprout Pharmaceuticals helped launch a public relations campaign called "Even the Score" which accused the FDA of sexism for only having ever approved drugs to treat male sexual dysfunction.
"The FDA has turned this down twice before, and now we have no new evidence of benefits, and we have more evidence of harms, so if the FDA does approve the drug today the only thing that changed is the amount of pressure put on it by a corporation though a major PR campaign," said Fugh-Berman.
Amy Allina, deputy director of the National Women’s Health Network said that while she agrees with the pharmaceutical company that women deserve to have their sexual problems taken seriously – "we do not believe that a minimally effective drug that must be taken daily, causes significant side effects and has not been evaluated for long-term safety, offers women a serious solution."