Sanofi: the new protein-based Covid vaccine to be used in the UK
Protein-based vaccines have been safely used for years against hep B, flu and shingles

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The University of Edinburgh’s Neil Mabbott on the vaccine technology being used for Covid booster jabs from this month.
In the UK a spring booster campaign has recently begun, offering an additional vaccine to people at highest risk from Covid. Between April and June 2023 people aged over 75, residents in care homes and children and adults aged five and up with weakened immune systems will be invited to get their next shot.
For some this will be their sixth dose to date. But as immunity from Covid vaccines begins to wane in the months afterwards, this is a sensible precaution to help protect the most vulnerable against serious illness, hospitalisation and death should they contract the virus.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
During this vaccination campaign the UK will be using the updated bivalent vaccines from Pfizer and Moderna (which target omicron alongside the original Covid strain) as well as another type of vaccine made by Sanofi.
The Sanofi vaccine was approved for use in the UK and the EU at the end of 2022. It’s the seventh Covid vaccine to receive approval in the UK.
Over the past couple of years we’ve heard plenty about Pfizer and Moderna’s mRNA-based vaccines. They’ve dominated the Covid vaccination campaign, particularly in high-income countries, with hundreds of millions of doses administered.
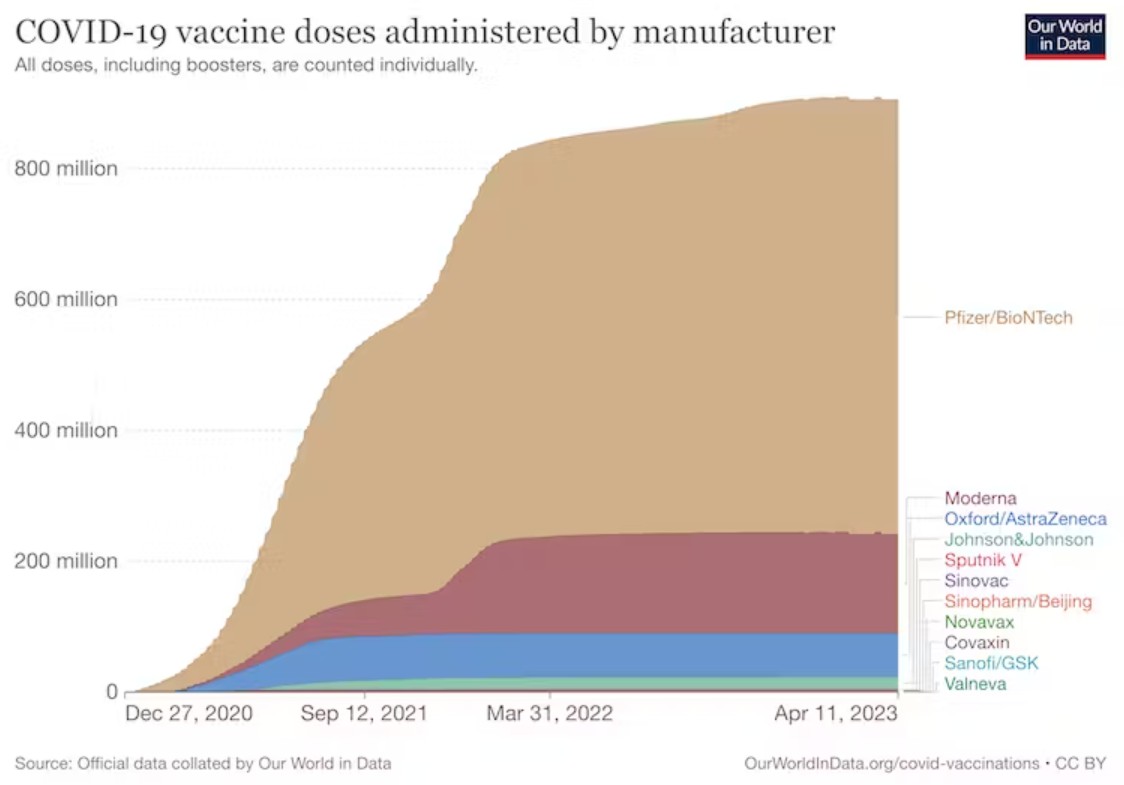
But the Sanofi booster is based on a different vaccine technology, which hasn’t been used before in a Covid booster campaign in the UK. So what is it, and how effective is this shot?
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
A protein-based vaccine
The Pfizer and Moderna vaccines contain mRNA, a small strand of genetic material that provides the instructions our cells need to make proteins. The mRNA instructs our cells to safely make copies of the spike protein which covers the surface of SARS-CoV-2 (the virus that causes Covid) and which helps it attach to and infect our cells. This trains our immune systems to recognise and kill SARS-CoV-2 if we encounter it.
The Sanofi vaccine, known as VidPrevtyn Beta, is protein-based and instead contains copies of the SARS-CoV-2 spike protein from the beta variant. This variant was first detected in South Africa and was classified as a variant of concern in December 2020.
So rather than instructing our cells to make copies of the spike protein as the mRNA vaccines do, the Sanofi vaccine contains copies of the spike protein already. Although the method is different, it delivers much the same effect – training our immune systems to recognise and kill SARS-CoV-2.
The Sanofi vaccine also contains an extra ingredient called an adjuvant, which stimulates our immune system to improve the vaccine’s potency.
This type of vaccine design is not new. Several protein-based vaccines have been safely used for many years to provide immunity against the tetanus toxin as well as viral diseases such as hepatitis B, human papillomavirus, influenza and shingles.
Other Covid vaccines have also used this approach, including one made by Novavax that was approved in the UK in February 2022. However, due to a number of reasons including delays in production compared to other manufacturers, the Novavax vaccine has not been used here.
Efficacy and safety
Early results from phase 3 trials indicate that the Sanofi vaccine, when used as a booster, is effective in generating strong virus-killing neutralising antibody responses against different Covid variants, including omicron. We call this cross protection, and it’s important to help provide immunity against any future variants of concern.
How long the immunity from this vaccine may last is uncertain at this stage, but – in studies in animals – neutralising antibody responses to different variants were maintained for up to six months.
No serious adverse reactions or safety concerns have been reported in clinical trials. Side effects, if encountered, were generally mild to moderate and occurred within the first few days. These included headache, muscle and joint pains, fever, nausea and pain or swelling at the injection site.
Mixing and matching
Using different Covid vaccines at the same time will ensure sufficient stocks are available to complete the current booster campaign as quickly as possible.
If you’re worried about getting a different vaccine to the one you had last time, there’s no need. Mixing and matching the different types of vaccines used for the initial shots and subsequent boosters is known as heterologous vaccination. This has already been happening in the UK – many people will have had a mixture of AstraZeneca, Pfizer or Moderna vaccinations over the past two years.
Heterologous vaccination can actually strengthen and broaden the immune response generated by the vaccine, helping to provide cross protection against different variants. In future booster campaigns we’ll probably continue to see a range of different types of Covid vaccines offered.
Comparing the options
The simplicity of the mRNA vaccine technology has enabled these shots to be rapidly updated to deal with emerging variants such as omicron. Protein-based vaccines tend to take longer to manufacture.
But they can be much cheaper to produce and can be stored in a refrigerator, unlike the mRNA vaccines which need to be stored in a freezer. These are useful characteristics to help improve vaccine equity in low and middle-income countries where vaccination coverage remains patchy.
Making direct comparisons between different vaccines and their effectiveness is always difficult, but the Sanofi booster appears to perform well when compared against the mRNA vaccines.
So if you’re medically vulnerable and are offered a booster vaccination it’s important that you take up this opportunity. Whether you’re given a protein-based vaccine or an mRNA-based vaccine, each will provide a good boost to your immunity that will help to protect you against serious illness with Covid.
Neil Mabbott, Personal Chair of Immunopathology, The University of Edinburgh
This article is republished from The Conversation under a Creative Commons license. Read the original article.
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 What is the endgame in the DHS shutdown?
What is the endgame in the DHS shutdown?Today’s Big Question Democrats want to rein in ICE’s immigration crackdown
-
 ‘Poor time management isn’t just an inconvenience’
‘Poor time management isn’t just an inconvenience’Instant Opinion Opinion, comment and editorials of the day
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
 How dangerous is the ‘K’ strain super-flu?
How dangerous is the ‘K’ strain super-flu?The Explainer Surge in cases of new variant H3N2 flu in UK and around the world
-
 Vaccine critic quietly named CDC’s No. 2 official
Vaccine critic quietly named CDC’s No. 2 officialSpeed Read Dr. Ralph Abraham joins another prominent vaccine critic, HHS Secretary Robert F. Kennedy Jr.
-
 This flu season could be worse than usual
This flu season could be worse than usualIn the spotlight A new subvariant is infecting several countries
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system