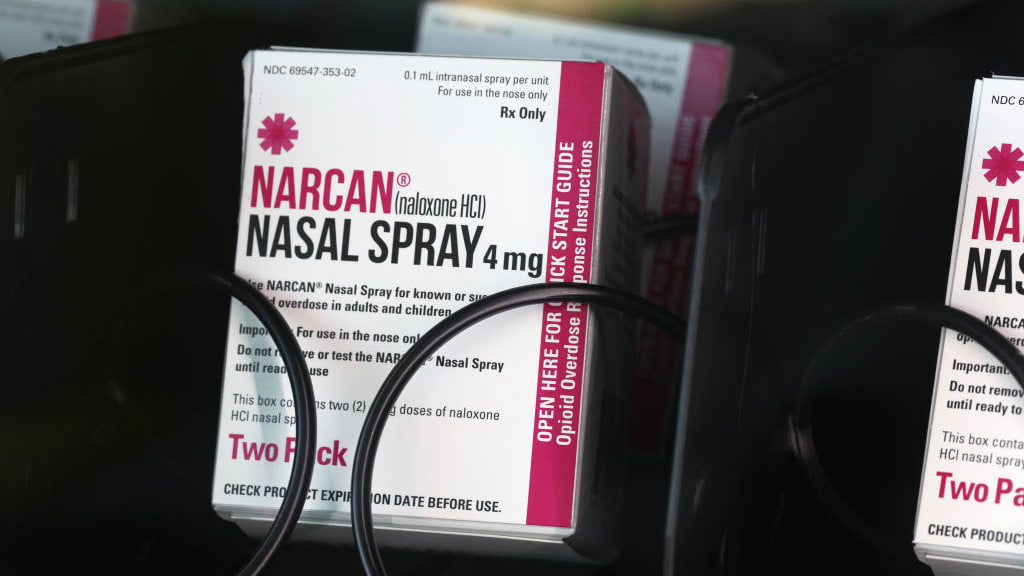
FDA advisers recommend making Narcan available over the counter

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Independent advisers to the Food and Drug Administration on Wednesday unanimously recommended over-the-counter sales of Narcan, a nasal spray that can reverse opioid overdoses.
Narcan, known generically as naloxone, blocks the effects of opiates on the brain and is usually administered by first responders and outreach workers. Many public health experts have argued that it needs to be widely available without a prescription so people who use drugs, their friends, and relatives can have easy access to it. In 2021, there were 107,000 fatal drug overdoses in the United States, including several people who died after illegally buying pills like Xanax and Percocet that were laced with the synthetic opioid fentanyl.
The advisers determined that Narcan requires no training to administer and is "abundantly safe and effective even in infants, with almost no potential for misuse or abuse," The New York Times writes. Because of their unanimous vote, it's likely the FDA will approve an over-the-counter version of the medication in March, meaning it could hit store shelves and vending machines by summer.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The advisers all being in agreement "underscores the importance of moving this drug to greater access and also highlights the terrible risk of not acting in terms of making the drug more accessible," Maris C. Coyle, chairwoman of the advisory panel and an associate clinical professor at the Ohio State University College of Pharmacy, told the Times.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Catherine Garcia has worked as a senior writer at The Week since 2014. Her writing and reporting have appeared in Entertainment Weekly, The New York Times, Wirecutter, NBC News and "The Book of Jezebel," among others. She's a graduate of the University of Redlands and the Columbia University Graduate School of Journalism.
-
 The Olympic timekeepers keeping the Games on track
The Olympic timekeepers keeping the Games on trackUnder the Radar Swiss watchmaking giant Omega has been at the finish line of every Olympic Games for nearly 100 years
-
 Will increasing tensions with Iran boil over into war?
Will increasing tensions with Iran boil over into war?Today’s Big Question President Donald Trump has recently been threatening the country
-
 Corruption: The spy sheikh and the president
Corruption: The spy sheikh and the presidentFeature Trump is at the center of another scandal
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Nitazene is quietly increasing opioid deaths
Nitazene is quietly increasing opioid deathsThe explainer The drug is usually consumed accidentally
-
 Can TrumpRx really lower drug prices?
Can TrumpRx really lower drug prices?Today’s Big Question Pfizer’s deal with Trump sent drugmaker stocks higher
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 The UK’s opioid crisis: why the stats don’t add up
The UK’s opioid crisis: why the stats don’t add upThe Explainer A new report has revealed that the UK’s total of opioid-related deaths could be much greater than official figures show
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
