FDA approves inhalable diabetes medication


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Three years after the Food and Drug Administration told MannKind Corp. to run additional tests on Afrezza, the inhalable diabetes medication has finally been approved. Afrezza is an insulin powder that users inhale from a single-use cartridge before or within 20 minutes of eating a meal, The Associated Press reports.
It is meant for those suffering from type 2 diabetes, the most common type of the disease, which stops the body from properly using insulin. As obesity rates soar worldwide, so too have the rates of those suffering from the chronic condition. Several other inhalable insulin medications have tanked either on the market or in development, including one from Pfizer Inc. in 2007 and another by Eli Lilly & Co. a year later. The FDA has noted that Afrezza should not be used as a long-term alternative to longer-acting insulin injections.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Sarah Eberspacher is an associate editor at TheWeek.com. She has previously worked as a sports reporter at The Livingston County Daily Press & Argus and The Arizona Republic. She graduated from Northwestern University's Medill School of Journalism.
-
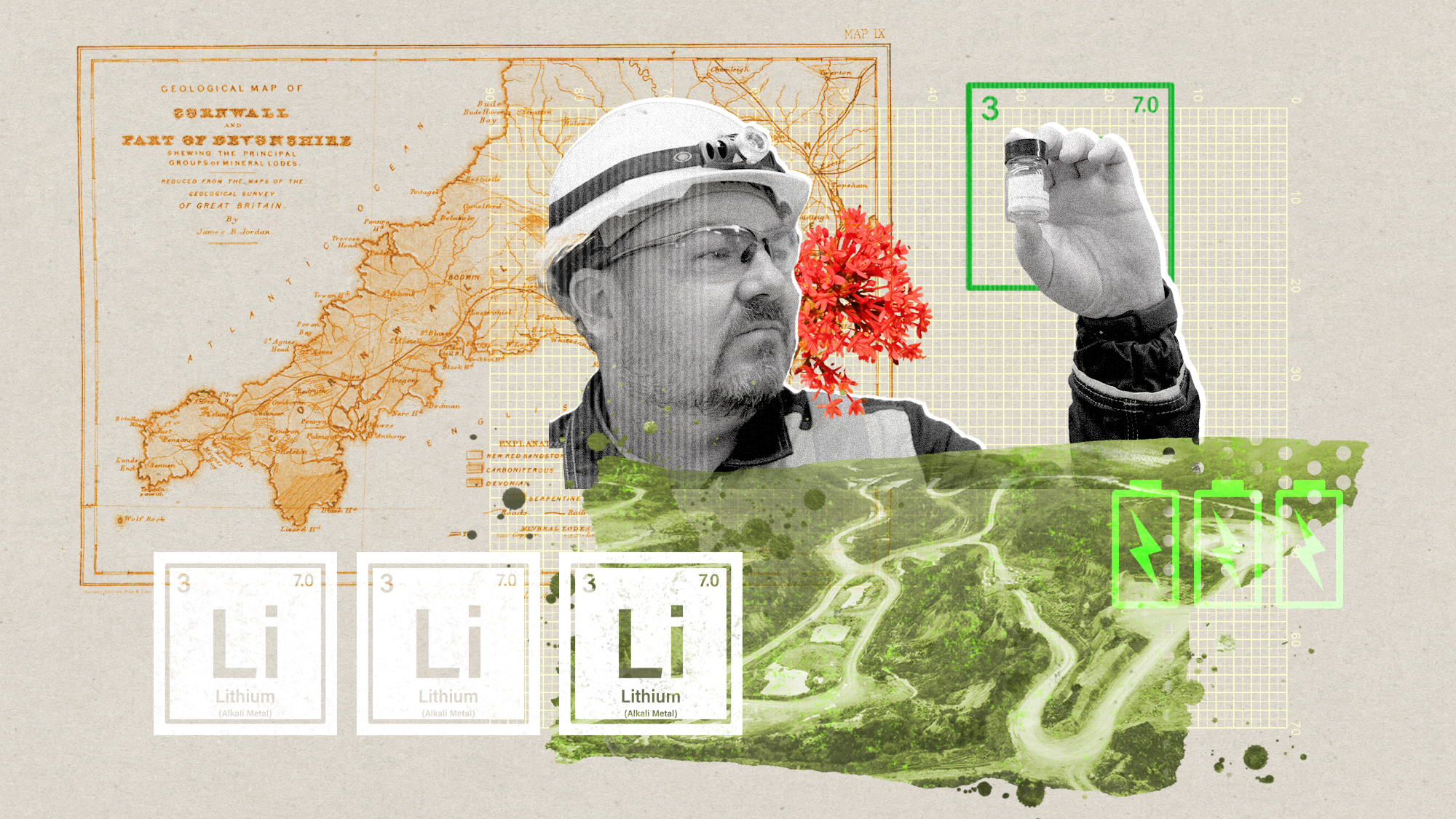 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 Nobody seems surprised Wagner's Prigozhin died under suspicious circumstances
Nobody seems surprised Wagner's Prigozhin died under suspicious circumstancesSpeed Read
-
 Western mountain climbers allegedly left Pakistani porter to die on K2
Western mountain climbers allegedly left Pakistani porter to die on K2Speed Read
-
 'Circular saw blades' divide controversial Rio Grande buoys installed by Texas governor
'Circular saw blades' divide controversial Rio Grande buoys installed by Texas governorSpeed Read
-
 Los Angeles city workers stage 1-day walkout over labor conditions
Los Angeles city workers stage 1-day walkout over labor conditionsSpeed Read
-
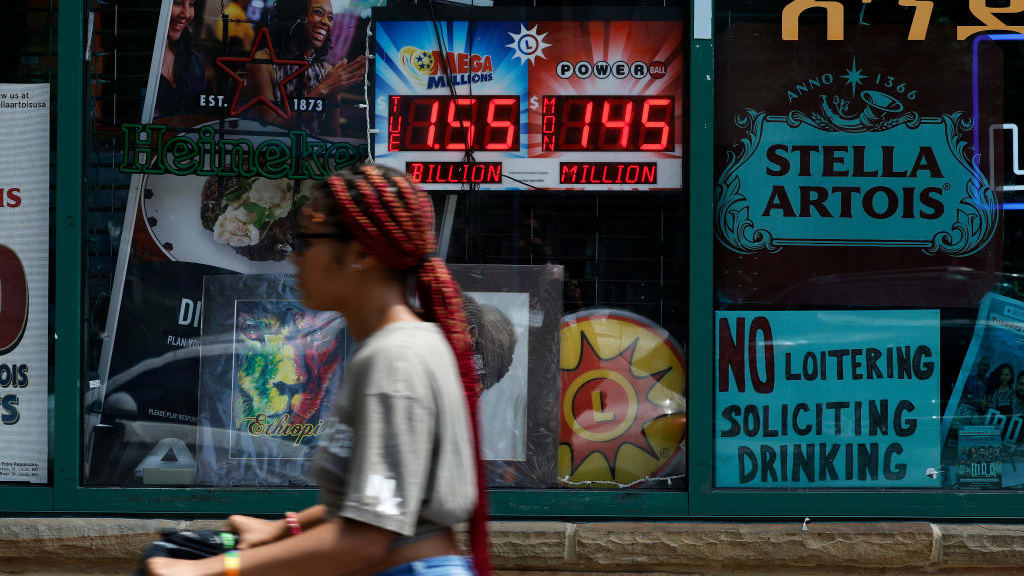 Mega Millions jackpot climbs to an estimated $1.55 billion
Mega Millions jackpot climbs to an estimated $1.55 billionSpeed Read
-
 Bangladesh dealing with worst dengue fever outbreak on record
Bangladesh dealing with worst dengue fever outbreak on recordSpeed Read
-
 Glacial outburst flooding in Juneau destroys homes
Glacial outburst flooding in Juneau destroys homesSpeed Read
-
 Scotland seeking 'monster hunters' to search for fabled Loch Ness creature
Scotland seeking 'monster hunters' to search for fabled Loch Ness creatureSpeed Read
