FDA approves revolutionary new cell-modifying cancer treatment
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
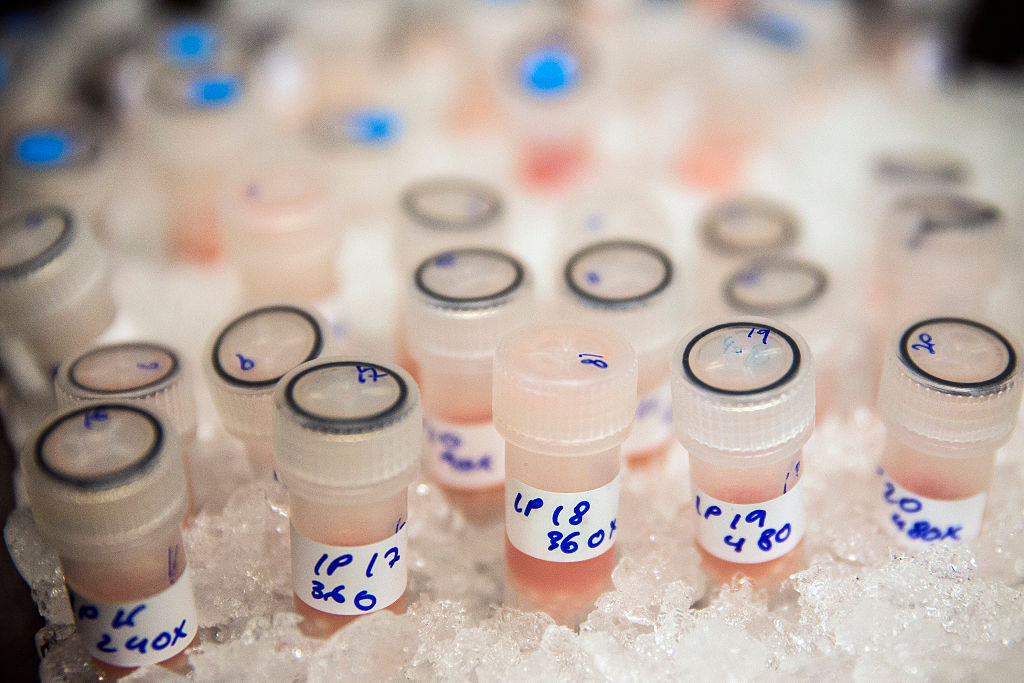
On Wednesday, the Food and Drug Administration approved a revolutionary new type of cancer treatment that genetically modifies the body's own cells. The treatment, known as CAR-T therapy, is the first type of gene therapy approved in the U.S and will be used to attack one of the most common childhood cancers, acute lymphoblastic leukemia.
The treatment first requires researchers to sift T cells from a patient's blood. The T cells, key in producing the body's immune response, are then reprogrammed in the lab with the addition of a receptor, called the CAR (chimeric antigen receptor), that helps T cells better find and fight cancer cells. After the laboratory work, the modified cells are inserted back into the patient, where they get to work.
Ted Laetsch, a doctor at the University of Texas Southwestern Medical Center, said that while the long-term success of the treatment is still being studied, a "far higher percentage of patients go into remission with this therapy than anything else we've seen to date with relapsed leukemia." One study found that 83 percent of advanced patients went into remission after receiving the treatment.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"We're entering a new frontier in medical innovation with the ability to reprogram a patient's own cells to attack a deadly cancer," FDA Commissioner Scott Gottlieb said in a statement. "New technologies such as gene and cell therapies hold out the potential to transform medicine and create an inflection point in our ability to treat and even cure many intractable illnesses."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
-
 The Olympic timekeepers keeping the Games on track
The Olympic timekeepers keeping the Games on trackUnder the Radar Swiss watchmaking giant Omega has been at the finish line of every Olympic Games for nearly 100 years
-
 Will increasing tensions with Iran boil over into war?
Will increasing tensions with Iran boil over into war?Today’s Big Question President Donald Trump has recently been threatening the country
-
 Corruption: The spy sheikh and the president
Corruption: The spy sheikh and the presidentFeature Trump is at the center of another scandal
-
 Nobody seems surprised Wagner's Prigozhin died under suspicious circumstances
Nobody seems surprised Wagner's Prigozhin died under suspicious circumstancesSpeed Read
-
 Western mountain climbers allegedly left Pakistani porter to die on K2
Western mountain climbers allegedly left Pakistani porter to die on K2Speed Read
-
 'Circular saw blades' divide controversial Rio Grande buoys installed by Texas governor
'Circular saw blades' divide controversial Rio Grande buoys installed by Texas governorSpeed Read
-
 Los Angeles city workers stage 1-day walkout over labor conditions
Los Angeles city workers stage 1-day walkout over labor conditionsSpeed Read
-
 Mega Millions jackpot climbs to an estimated $1.55 billion
Mega Millions jackpot climbs to an estimated $1.55 billionSpeed Read
-
 Bangladesh dealing with worst dengue fever outbreak on record
Bangladesh dealing with worst dengue fever outbreak on recordSpeed Read
-
 Glacial outburst flooding in Juneau destroys homes
Glacial outburst flooding in Juneau destroys homesSpeed Read
-
 Scotland seeking 'monster hunters' to search for fabled Loch Ness creature
Scotland seeking 'monster hunters' to search for fabled Loch Ness creatureSpeed Read