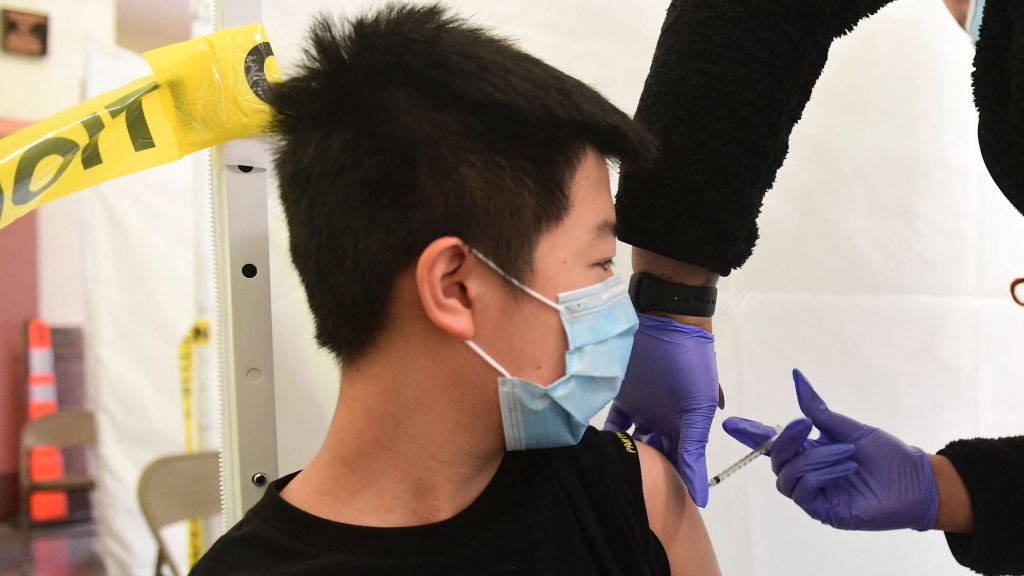
FDA backs Pfizer COVID booster for 12- to 15-year-olds

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The U.S. Food and Drug Administration on Monday officially authorized Pfizer-BioNTech COVID-19 boosters for use in children ages 12 to 15, The Washington Post reports, "an effort to bolster protection as schools reopen amid a surge of infections caused by the Omicron variant."
According to CNBC, the FDA also cut down the recommended time between primary vaccination and an individual's booster dose from six months to at least five, and authorized a "third vaccine dose as part of the primary series of shots for children ages 5 through 11 who have compromised immune systems."
The agency's verdict arrives as schools decide whether and how to reopen schools while an Omicron surge tears its way through the country, per the Post. According to the Centers for Disease Control and Prevention, about one-third of eligible Americans have thus far received a COVID booster shot.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"The recent rise in COVID-19 cases is concerning to all and today's decision by the FDA to further expand the Emergency Use Authorization of a booster dose of our vaccine is critical to help us ultimately defeat this pandemic," said Pfizer CEO Albert Bourla in a statement.
The expansion will now be reviewed by the CDC and its panel of vaccine advisers likely later this week, notes the Post. Should the outside committee sign off on the additional shots, "CDC director Rochelle Walensky is expected to officially recommend them" that same day.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brigid Kennedy worked at The Week from 2021 to 2023 as a staff writer, junior editor and then story editor, with an interest in U.S. politics, the economy and the music industry.
-
 6 exquisite homes with vast acreage
6 exquisite homes with vast acreageFeature Featuring an off-the-grid contemporary home in New Mexico and lakefront farmhouse in Massachusetts
-
 Film reviews: ‘Wuthering Heights,’ ‘Good Luck, Have Fun, Don’t Die,’ and ‘Sirat’
Film reviews: ‘Wuthering Heights,’ ‘Good Luck, Have Fun, Don’t Die,’ and ‘Sirat’Feature An inconvenient love torments a would-be couple, a gonzo time traveler seeks to save humanity from AI, and a father’s desperate search goes deeply sideways
-
 Political cartoons for February 16
Political cartoons for February 16Cartoons Monday’s political cartoons include President's Day, a valentine from the Epstein files, and more
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 The new Stratus Covid strain – and why it’s on the rise
The new Stratus Covid strain – and why it’s on the riseThe Explainer ‘No evidence’ new variant is more dangerous or that vaccines won’t work against it, say UK health experts
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
