New Alzheimer's drug rejected: is Nice being nasty?
Health watchdog has announced lecanemab will be denied to NHS patients on cost grounds
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
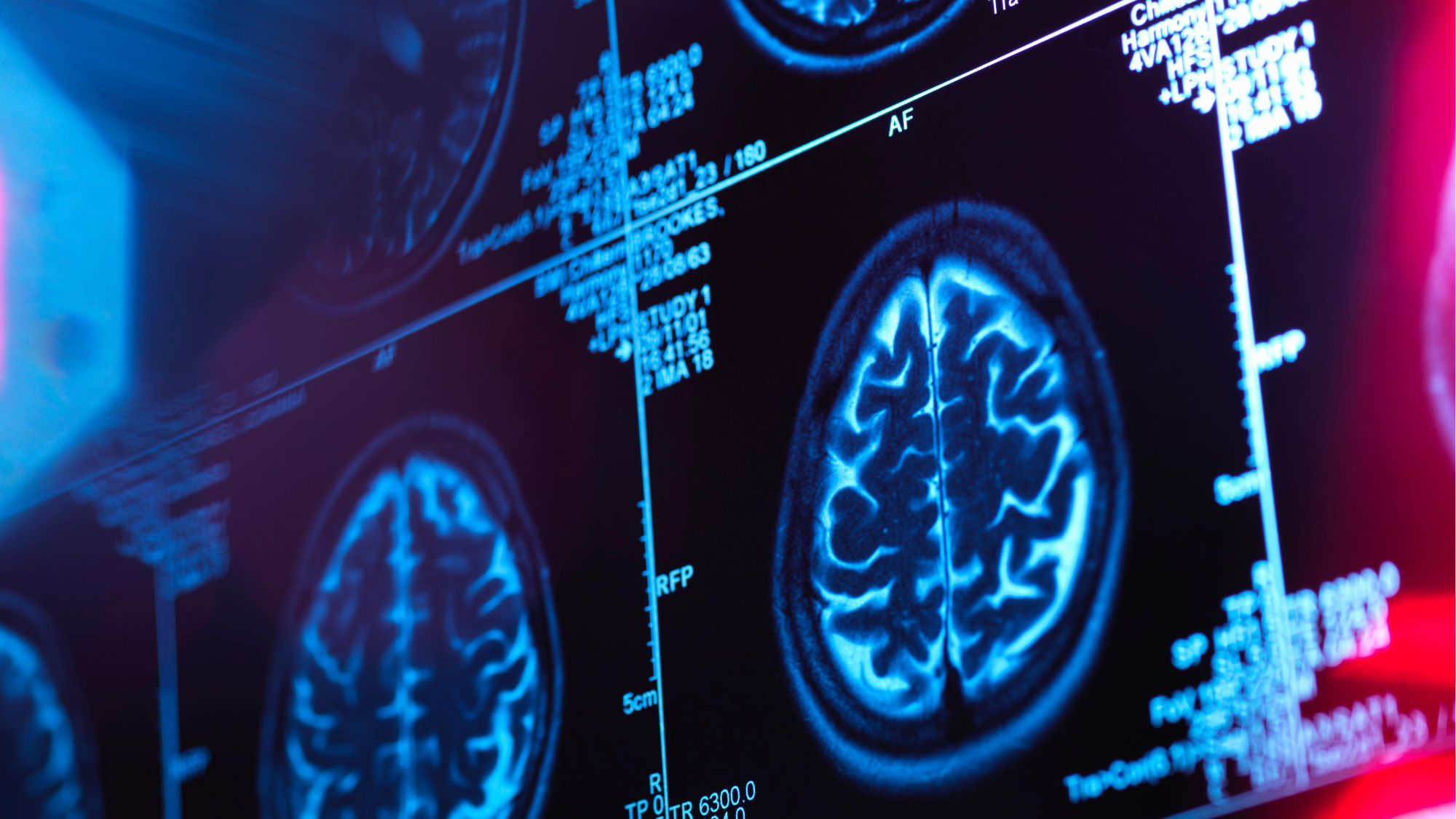
"I am personally familiar with the horrors of Alzheimer's," said Catherine Pepinster in The Daily Telegraph: it's a disease my mother suffered from. So I was delighted to hear last week that the first drug found to slow down the onset of the condition had been approved for use in the UK. No sooner had this good news emerged, though, than the health watchdog Nice announced that the drug would be denied to NHS patients on cost grounds.
Trials have shown that 70,000 people in England with early-stage Alzheimer's might have been eligible for treatment with lecanemab, the drug in question, at a cost of £30,000 a year per patient – adding up to a total potential bill of some £2.1bn. Granted, that's a lot of money, but the NHS spends more than three times as much each year treating obesity. "Expensive surgeries like gastric bypasses are not withheld from the morbidly overweight. So why is a drug that could transform elderly lives neglected?"
At first glance, this may seem like "a kick in the teeth for patients", said Clare Wilson on the i news site, but Nice's decision is not driven by mere penny-pinching. For one thing, the benefits from lecanemab – which has to be administered through fortnightly infusions in hospital – are comparatively modest. The clinical trials involved giving patients complex memory tests and assessments, scoring them on a scale from nought to 18, with higher scores meaning worse memory problems. After 18 months, the scores of people receiving placebo infusions rose by an average of 1.7 points, while the scores of those on lecanemab rose by 1.2 points – not much of a difference. The drug can also cause side effects such as small brain bleeds. The money it would cost to provide lecanemab on the NHS would be better spent on treating Alzheimer's patients' symptoms, or providing sufferers with more support from social services and home nurses.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
A balance clearly has to be struck with every drug, said The Independent, but it's sad that only those who can afford to go private stand to benefit from this one. Any delay in the progression of Alzheimer's is priceless for patients – and for their families, whose interests did not feature in Nice's cost-benefit analysis. The sole consolation is that lecanemab is likely to be the first of many such drugs to appear on the market, whose costs are likely to fall over time.
In fact NHS England is currently considering 27 other Alzheimer's drugs, said Oliver Duff on the i news site: these are in advanced trials and could well be approved in the next few years. "Humanity is making progress against this appalling disease, finally unravelling the mysteries of the brain."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
-
 Why is the Trump administration talking about ‘Western civilization’?
Why is the Trump administration talking about ‘Western civilization’?Talking Points Rubio says Europe, US bonded by religion and ancestry
-
 Quentin Deranque: a student’s death energizes the French far right
Quentin Deranque: a student’s death energizes the French far rightIN THE SPOTLIGHT Reactions to the violent killing of an ultraconservative activist offer a glimpse at the culture wars roiling France ahead of next year’s elections
-
 Secured vs. unsecured loans: how do they differ and which is better?
Secured vs. unsecured loans: how do they differ and which is better?the explainer They are distinguished by the level of risk and the inclusion of collateral
-
 Growing a brain in the lab
Growing a brain in the labFeature It's a tiny version of a developing human cerebral cortex
-
 Mixed nuts: RFK Jr.’s new nutrition guidelines receive uneven reviews
Mixed nuts: RFK Jr.’s new nutrition guidelines receive uneven reviewsTalking Points The guidelines emphasize red meat and full-fat dairy
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
 Obesity drugs: Will Trump’s plan lower costs?
Obesity drugs: Will Trump’s plan lower costs?Feature Even $149 a month, the advertised price for a starting dose of a still-in-development GLP-1 pill on TrumpRx, will be too big a burden for the many Americans ‘struggling to afford groceries’
-
 Ultra-processed America
Ultra-processed AmericaFeature Highly processed foods make up most of our diet. Is that so bad?
-
 The quest to defy ageing
The quest to defy ageingThe Explainer Humanity has fantasised about finding the fountain of youth for millennia. How close are we now?
-
 The battle of the weight-loss drugs
The battle of the weight-loss drugsTalking Point Can Novo Nordisk and Eli Lilly regain their former stock market glory? A lot is riding on next year's pills
-
 An insatiable hunger for protein
An insatiable hunger for proteinFeature Americans can't get enough of the macronutrient. But how much do we really need?