Here's what happens when you put a can of soda in liquid nitrogen


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Nitrogen boils at -320 degrees Fahrenheit, and liquid nitrogen is even colder than that. So when you place an aluminum vessel full of soft drink inside liquid nitrogen, two things should happen. First, carbon dioxide is not very soluble in ice, so when the soda freezes, the can should burst. Second, any ice created should be in very small chunks, because the rapid temperature change will cause it to crack many times.
Does it actually work? Watch to find out. Ryan Cooper

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Ryan Cooper is a national correspondent at TheWeek.com. His work has appeared in the Washington Monthly, The New Republic, and the Washington Post.
-
 The environmental cost of GLP-1s
The environmental cost of GLP-1sThe explainer Producing the drugs is a dirty process
-
 Greenland’s capital becomes ground zero for the country’s diplomatic straits
Greenland’s capital becomes ground zero for the country’s diplomatic straitsIN THE SPOTLIGHT A flurry of new consular activity in Nuuk shows how important Greenland has become to Europeans’ anxiety about American imperialism
-
 ‘This is something that happens all too often’
‘This is something that happens all too often’Instant Opinion Opinion, comment and editorials of the day
-
 Nobody seems surprised Wagner's Prigozhin died under suspicious circumstances
Nobody seems surprised Wagner's Prigozhin died under suspicious circumstancesSpeed Read
-
 Western mountain climbers allegedly left Pakistani porter to die on K2
Western mountain climbers allegedly left Pakistani porter to die on K2Speed Read
-
 'Circular saw blades' divide controversial Rio Grande buoys installed by Texas governor
'Circular saw blades' divide controversial Rio Grande buoys installed by Texas governorSpeed Read
-
 Los Angeles city workers stage 1-day walkout over labor conditions
Los Angeles city workers stage 1-day walkout over labor conditionsSpeed Read
-
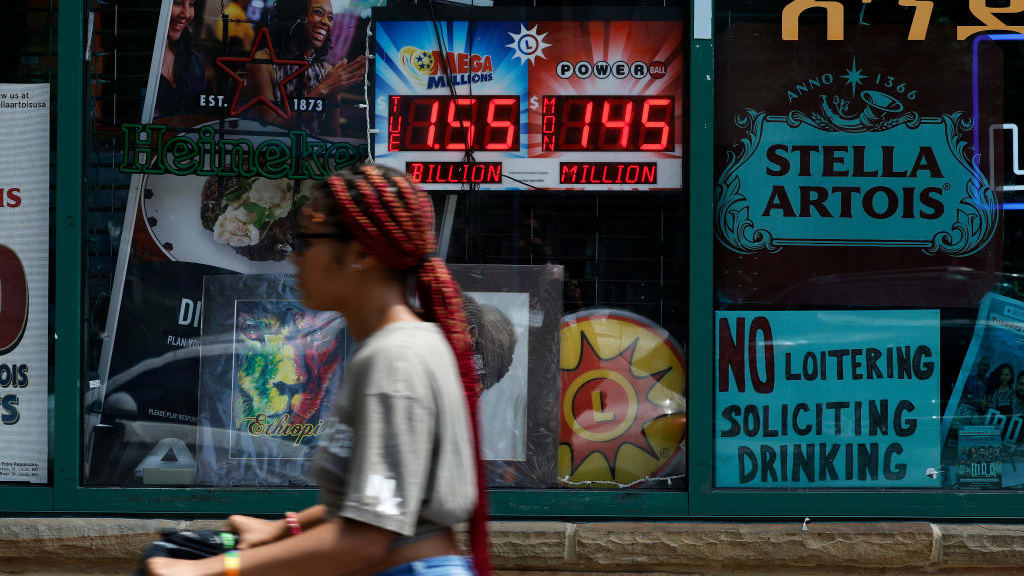 Mega Millions jackpot climbs to an estimated $1.55 billion
Mega Millions jackpot climbs to an estimated $1.55 billionSpeed Read
-
 Bangladesh dealing with worst dengue fever outbreak on record
Bangladesh dealing with worst dengue fever outbreak on recordSpeed Read
-
 Glacial outburst flooding in Juneau destroys homes
Glacial outburst flooding in Juneau destroys homesSpeed Read
-
 Scotland seeking 'monster hunters' to search for fabled Loch Ness creature
Scotland seeking 'monster hunters' to search for fabled Loch Ness creatureSpeed Read
