FDA approves first gene therapy for adults with blood cancer

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
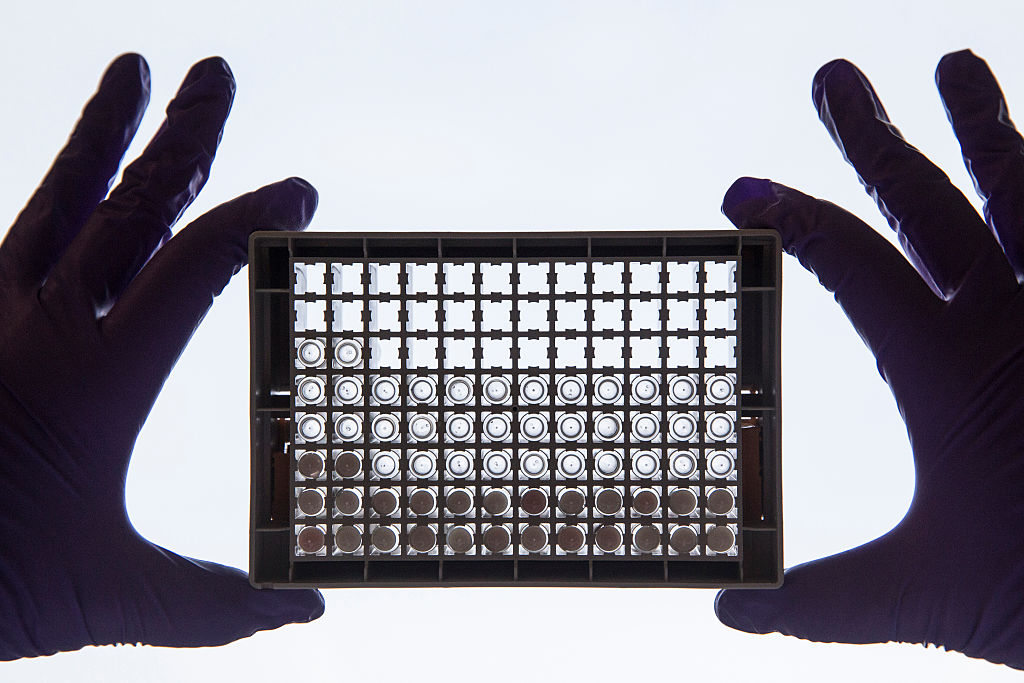
The Food and Drug Administration on Wednesday approved a one-time treatment for lymphoma in adults, only the second time a gene therapy for blood cancer has been given the okay in the United States.
This is the first gene therapy approved for adults, and it involves removing a patient's T cells, reprogramming them to find and kill cancer cells, then putting the cells back into the patient, The Associated Press reports. The treatment uses the same technology as a gene therapy recently approved in the U.S. for childhood leukemia, and will cost $373,000 per patient, its manufacturer said.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Catherine Garcia has worked as a senior writer at The Week since 2014. Her writing and reporting have appeared in Entertainment Weekly, The New York Times, Wirecutter, NBC News and "The Book of Jezebel," among others. She's a graduate of the University of Redlands and the Columbia University Graduate School of Journalism.
-
 Tourangelle-style pork with prunes recipe
Tourangelle-style pork with prunes recipeThe Week Recommends This traditional, rustic dish is a French classic
-
 The Epstein files: glimpses of a deeply disturbing world
The Epstein files: glimpses of a deeply disturbing worldIn the Spotlight Trove of released documents paint a picture of depravity and privilege in which men hold the cards, and women are powerless or peripheral
-
 Jeff Bezos: cutting the legs off The Washington Post
Jeff Bezos: cutting the legs off The Washington PostIn the Spotlight A stalwart of American journalism is a shadow of itself after swingeing cuts by its billionaire owner
-
 TikTok secures deal to remain in US
TikTok secures deal to remain in USSpeed Read ByteDance will form a US version of the popular video-sharing platform
-
 Unemployment rate ticks up amid fall job losses
Unemployment rate ticks up amid fall job lossesSpeed Read Data released by the Commerce Department indicates ‘one of the weakest American labor markets in years’
-
 US mints final penny after 232-year run
US mints final penny after 232-year runSpeed Read Production of the one-cent coin has ended
-
 Warner Bros. explores sale amid Paramount bids
Warner Bros. explores sale amid Paramount bidsSpeed Read The media giant, home to HBO and DC Studios, has received interest from multiple buying parties
-
 Gold tops $4K per ounce, signaling financial unease
Gold tops $4K per ounce, signaling financial uneaseSpeed Read Investors are worried about President Donald Trump’s trade war
-
 Electronic Arts to go private in record $55B deal
Electronic Arts to go private in record $55B dealspeed read The video game giant is behind ‘The Sims’ and ‘Madden NFL’
-
 New York court tosses Trump's $500M fraud fine
New York court tosses Trump's $500M fraud fineSpeed Read A divided appeals court threw out a hefty penalty against President Trump for fraudulently inflating his wealth
-
 Trump said to seek government stake in Intel
Trump said to seek government stake in IntelSpeed Read The president and Intel CEO Lip-Bu Tan reportedly discussed the proposal at a recent meeting
