Tuberculosis vaccine in late-stage trial as COVID-19 protection


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Texas A&M College of Medicine is leading a consortium of research hospitals and medical schools in a Phase 4 trial to determine if the century-old tuberculosis vaccine can help blunt the damage from COVID-19, at least until a vaccine for the new coronavirus has been proven safe and effective.
"Scientists have known for decades that the tuberculosis vaccine, called bacille Calmette-Guerin, or BCG, improves immunity against some viruses," The Texas Tribune reported back in May, when the trial was just getting started. Jeffrey Cirillo, the Texas A&M microbial pathogenesis and immunology professor who is leading the trial, told Politico on Thursday that about 100 people have already been vaccinated, 200-300 more will get their shots over the next two weeks, and the goal is 1,800 subjects in the "randomized, blinded, placebo-controlled trial."
The TB vaccine has been used more than a billion times around the world, but it's not commonly used in the U.S., except to fight bladder cancer. The researchers at Texas A&M, Baylor College of Medicine, Harvard's School of Public Health, Cedars Sinai Medical Center, and the University of Texas MD Anderson Cancer Center are hoping the vaccine ramps up the immune system to fight off the disease, as it does the cancer cells. A similar trial is being conducted in the Netherlands.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The U.S. researchers will monitor the volunteers for six months, looking for statistically significant differences between those who get the BCG vaccine and the group that gets a placebo shot. "We're also doing a cognitive study in parallel to evaluate the cognitive effects of COVID-19," Cirillo told Politico's Myah Ward, using before-and-after MRIs and cognitive assessments to see if the vaccine reduces COVID-19's mental impairments. The vaccine is most effective in the first two to three years, he added, and if it is found to be effective, it could be used either as a stop-gap measure until a coronavirus vaccine arrives or in tandem with that vaccine to make it more effective.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
 Colbert, CBS spar over FCC and Talarico interview
Colbert, CBS spar over FCC and Talarico interviewSpeed Read The late night host said CBS pulled his interview with Democratic Texas state representative James Talarico over new FCC rules about political interviews
-
 The Week contest: AI bellyaching
The Week contest: AI bellyachingPuzzles and Quizzes
-
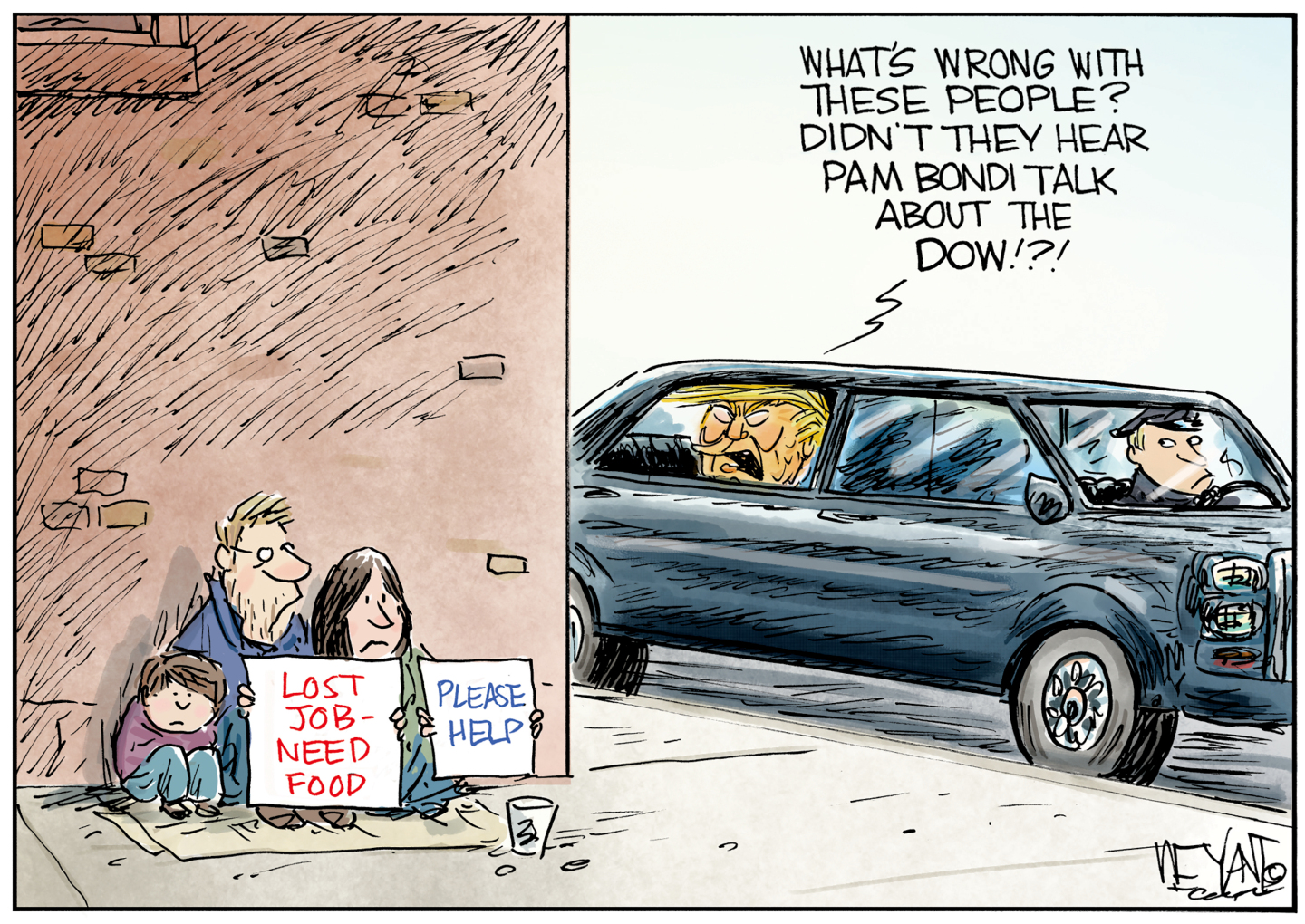 Political cartoons for February 18
Political cartoons for February 18Cartoons Wednesday’s political cartoons include the DOW, human replacement, and more
-
 TikTok secures deal to remain in US
TikTok secures deal to remain in USSpeed Read ByteDance will form a US version of the popular video-sharing platform
-
 Unemployment rate ticks up amid fall job losses
Unemployment rate ticks up amid fall job lossesSpeed Read Data released by the Commerce Department indicates ‘one of the weakest American labor markets in years’
-
 US mints final penny after 232-year run
US mints final penny after 232-year runSpeed Read Production of the one-cent coin has ended
-
 Warner Bros. explores sale amid Paramount bids
Warner Bros. explores sale amid Paramount bidsSpeed Read The media giant, home to HBO and DC Studios, has received interest from multiple buying parties
-
 Gold tops $4K per ounce, signaling financial unease
Gold tops $4K per ounce, signaling financial uneaseSpeed Read Investors are worried about President Donald Trump’s trade war
-
 Electronic Arts to go private in record $55B deal
Electronic Arts to go private in record $55B dealspeed read The video game giant is behind ‘The Sims’ and ‘Madden NFL’
-
 New York court tosses Trump's $500M fraud fine
New York court tosses Trump's $500M fraud fineSpeed Read A divided appeals court threw out a hefty penalty against President Trump for fraudulently inflating his wealth
-
 Trump said to seek government stake in Intel
Trump said to seek government stake in IntelSpeed Read The president and Intel CEO Lip-Bu Tan reportedly discussed the proposal at a recent meeting
