U.S. health officials say AstraZeneca may have skewed vaccine 'efficacy data' with 'outdated information'

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
In an unusual statement issued after midnight on Tuesday, the National Institute of Allergy and Infectious Diseases said an independent monitoring board overseeing AstraZeneca's U.S. COVID-19 vaccine trial told the NIAID and the drugmaker late Monday "it was concerned by information released by AstraZeneca on initial data from its COVID-19 vaccine clinical trial." The NIAID, a unit of the National Institutes of Health, is led by Dr. Anthony Fauci, President Biden's top medical adviser.
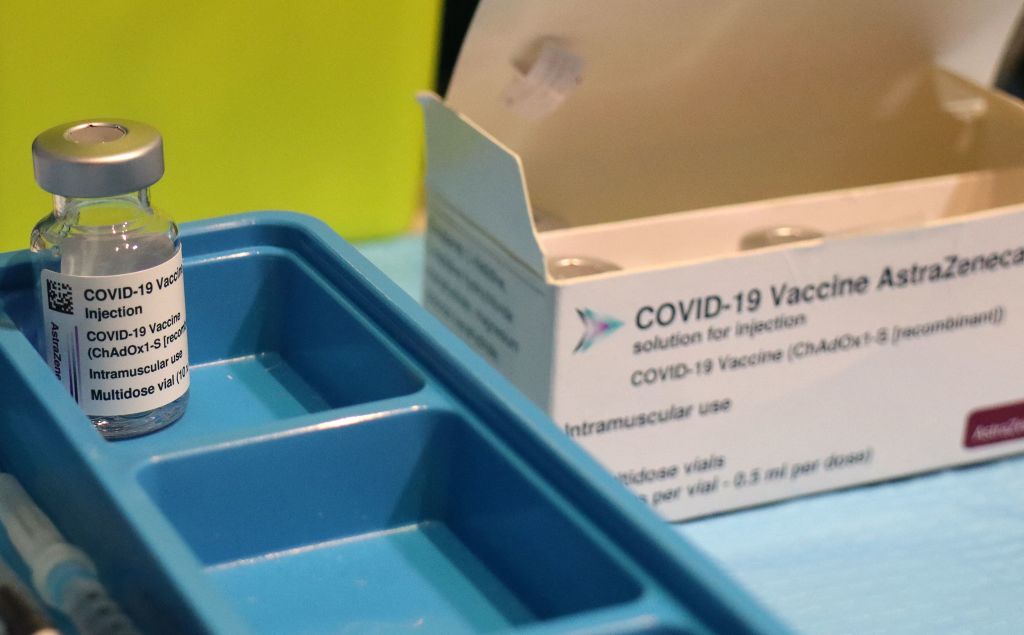
AstraZeneca reported early Monday that its vaccine had proved to be 79 percent effective against symptomatic COVID-19 in a large U.S. trial, 100 percent effective against serious illness or hospitalization, and carried no increased risk of blood clots. The results were seen as a shot in the arm for the beleaguered vaccine.
But the Data and Safety Monitoring Board (DSMB) "expressed concern that AstraZeneca may have included outdated information from that trial, which may have provided an incomplete view of the efficacy data," the NIAID said. The Food and Drug Administration and Centers for Disease Control and Prevention will ultimately conduct a thorough review of the data before approving AstraZeneca's vaccine for use in the U.S., the agency said, but AstraZeneca should "work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The DSMB's analysis of AstraZeneca's U.S.-based trial was "delayed several times because the board had to ask AstraZeneca for revised reports from those handling trial data on behalf of the company," The New York Times reports, citing a person familiar with the matter.
Friction between a safety monitoring board and a study sponsor is "highly irregular," and the NIAID's post-midnight statement is "so, so troubling," clinical trials expert Dr. Eric Topol told the Times. "I've never seen anything like this." AstraZeneca had yet to respond to the statement early Tuesday.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 TikTok secures deal to remain in US
TikTok secures deal to remain in USSpeed Read ByteDance will form a US version of the popular video-sharing platform
-
 Unemployment rate ticks up amid fall job losses
Unemployment rate ticks up amid fall job lossesSpeed Read Data released by the Commerce Department indicates ‘one of the weakest American labor markets in years’
-
 US mints final penny after 232-year run
US mints final penny after 232-year runSpeed Read Production of the one-cent coin has ended
-
 Warner Bros. explores sale amid Paramount bids
Warner Bros. explores sale amid Paramount bidsSpeed Read The media giant, home to HBO and DC Studios, has received interest from multiple buying parties
-
 Gold tops $4K per ounce, signaling financial unease
Gold tops $4K per ounce, signaling financial uneaseSpeed Read Investors are worried about President Donald Trump’s trade war
-
 Electronic Arts to go private in record $55B deal
Electronic Arts to go private in record $55B dealspeed read The video game giant is behind ‘The Sims’ and ‘Madden NFL’
-
 New York court tosses Trump's $500M fraud fine
New York court tosses Trump's $500M fraud fineSpeed Read A divided appeals court threw out a hefty penalty against President Trump for fraudulently inflating his wealth
-
 Trump said to seek government stake in Intel
Trump said to seek government stake in IntelSpeed Read The president and Intel CEO Lip-Bu Tan reportedly discussed the proposal at a recent meeting
