Why the FDA's decision on the morning-after pill will please no one
A modest change is being blasted as both too much and too little

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
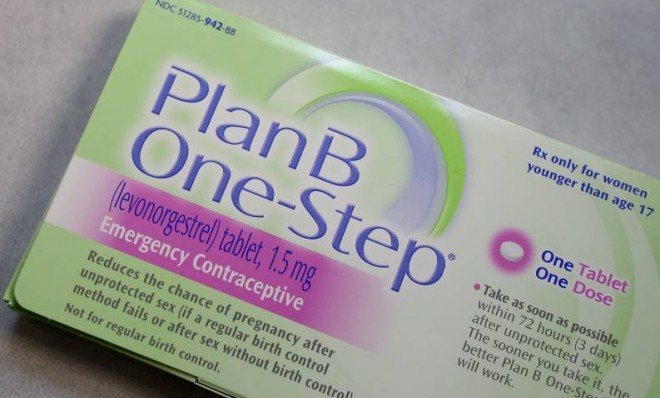
The Food and Drug Administration announced Tuesday that it would allow the morning-after pill to be sold without a prescription to women 15 and older, so long as they can provide legal proof of age. And no one is really happy about it.
Currently, the contraceptive is available over-the-counter to anyone over 17, but a court ruling mandating that it be available to all women regardless of age is set to kick in soon.
According to the FDA, the latest change is not in response to that pending legal action, but it falls right in between the two poles. And that means many parties have reason to be dissatisfied with the new policy, arguing either that the change is too much, or not enough.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
For one, it falls short of the unrestricted access favored by leading science and health groups like the American Medical Association. They're joined by liberal lawmakers, including Sen. Patty Murray (D-Wash.), who called the change a "step in the right direction," but said she would keep pushing for more open access. Planned Parenthood vowed to do the same, as did the Center for Reproductive Rights, which filed the lawsuit that led to the recent court ruling.
"Lowering the age restriction to 15 for over-the-counter access to Plan B One-Step may reduce delays for some young women — but it does nothing to address the significant barriers that far too many women of all ages will still find if they arrive at the drugstore without identification or after the pharmacy gates have been closed for the night or weekend," Nancy Northup, president and CEO of the Center for Reproductive Rights, said in a statement.
Social conservatives, meanwhile, are miffed that the FDA has granted wider access to a pill they oppose on principle.
"This ruling places the health of young girls at risk," a spokesperson for the Family Research Council told Life News. "Making Plan B available for girls under the age of 17 without a prescription flies in the face of medical information and sound judgment."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
The FDA's decision also falls short of its own stated position on the matter. In 2011, the FDA moved to grant unrestricted access to Plan B. However, the Department of Health and Human Services overruled that decision, keeping the age limit at 17. In permitting over-the-counter sales of Plan B to those 15 and up, the FDA has apparently pushed back against HHS and, by extension, the White House.
Then there's the court ruling. Earlier this month, a federal judge ordered the FDA to remove all restrictions on sales of Plan B within 30 days, so the latest change still leaves the FDA short of that mandate. The government has until May 5 to appeal the ruling or comply, but has yet to indicate which route it will take.
It seems the only party that got exactly what it wanted is the drug company TEVA Pharmaceuticals, whose application to sell Plan B the FDA was approved Tuesday.
Jon Terbush is an associate editor at TheWeek.com covering politics, sports, and other things he finds interesting. He has previously written for Talking Points Memo, Raw Story, and Business Insider.
-
 The Gallivant: style and charm steps from Camber Sands
The Gallivant: style and charm steps from Camber SandsThe Week Recommends Nestled behind the dunes, this luxury hotel is a great place to hunker down and get cosy
-
 The President’s Cake: ‘sweet tragedy’ about a little girl on a baking mission in Iraq
The President’s Cake: ‘sweet tragedy’ about a little girl on a baking mission in IraqThe Week Recommends Charming debut from Hasan Hadi is filled with ‘vivid characters’
-
 Kia EV4: a ‘terrifically comfy’ electric car
Kia EV4: a ‘terrifically comfy’ electric carThe Week Recommends The family-friendly vehicle has ‘plush seats’ and generous space
-
 The billionaires’ wealth tax: a catastrophe for California?
The billionaires’ wealth tax: a catastrophe for California?Talking Point Peter Thiel and Larry Page preparing to change state residency
-
 Bari Weiss’ ‘60 Minutes’ scandal is about more than one report
Bari Weiss’ ‘60 Minutes’ scandal is about more than one reportIN THE SPOTLIGHT By blocking an approved segment on a controversial prison holding US deportees in El Salvador, the editor-in-chief of CBS News has become the main story
-
 Has Zohran Mamdani shown the Democrats how to win again?
Has Zohran Mamdani shown the Democrats how to win again?Today’s Big Question New York City mayoral election touted as victory for left-wing populists but moderate centrist wins elsewhere present more complex path for Democratic Party
-
 Millions turn out for anti-Trump ‘No Kings’ rallies
Millions turn out for anti-Trump ‘No Kings’ ralliesSpeed Read An estimated 7 million people participated, 2 million more than at the first ‘No Kings’ protest in June
-
 Ghislaine Maxwell: angling for a Trump pardon
Ghislaine Maxwell: angling for a Trump pardonTalking Point Convicted sex trafficker's testimony could shed new light on president's links to Jeffrey Epstein
-
 The last words and final moments of 40 presidents
The last words and final moments of 40 presidentsThe Explainer Some are eloquent quotes worthy of the holders of the highest office in the nation, and others... aren't
-
 The JFK files: the truth at last?
The JFK files: the truth at last?In The Spotlight More than 64,000 previously classified documents relating the 1963 assassination of John F. Kennedy have been released by the Trump administration
-
 'Seriously, not literally': how should the world take Donald Trump?
'Seriously, not literally': how should the world take Donald Trump?Today's big question White House rhetoric and reality look likely to become increasingly blurred
