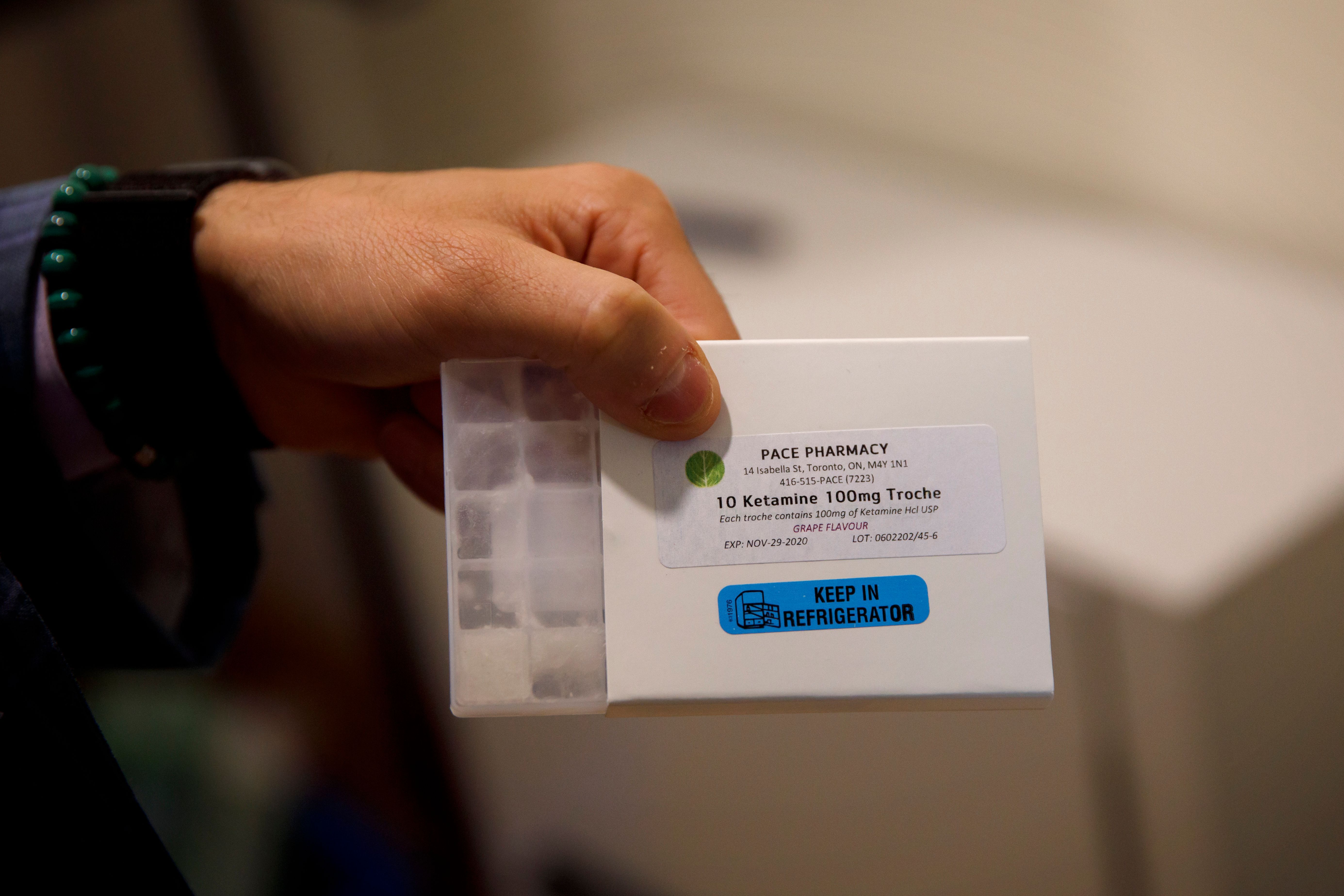
Is ketamine a safe at-home treatment for depression?
Loosened regulations have led to a proliferation of people taking the drug without medical supervision.

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Ketamine has been around for decades but has been having a resurgence in recent years as an option for people with treatment-resistant depression. Loosened regulations around remote prescriptions for controlled substances have led to a proliferation of people taking the drug without medical supervision. While many hail the drug as a miracle treatment, some medical experts are concerned there's not enough research to support making the drug so easily accessible.
Is using ketamine to treat depression legal?
Ketamine is a hallucinogen that has been approved for use as an anesthetic by the Food and Drug Administration since the 1970s. It's also known as a street drug abused for its mind-altering effects, under names like Special K. In recent years, "it has shown promise for delivering rapid relief to patients with mental health conditions who have tried conventional antidepressants without success," according to The Washington Post. Several research studies in recent years showed it had some promise as an off-label antidepressant.
In 2019, the FDA approved Spravato, "a nasal spray derived from ketamine," for treating severe depression. "But that approval came with strict guardrails to ensure patient safety," added the Post," a nod to known side effects such as altered consciousness and increases in blood pressure." The FDA requires that health care providers monitor patients for two hours after they take Spravato.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
While it's legal to prescribe ketamine for depression, "patients needed to first meet in person with a doctor, and treatment was mostly limited to infusions in clinics," reported The New York Times. Access to the drug was tightly controlled by the Drug Enforcement Administration, "which puts its risk of abuse one notch below that of opioids like oxycodone and fentanyl."
How are people getting access to ketamine at home?
In recent years, "even before the approval of Spravato" ketamine clinics, "which offer the drug off-label as either an infusion or an injection for a wide variety of mental health problems," started to pop up all over the United States, reported NBC News. The clinics had to abide by the FDA's regulations to administer the drug, including mandatory in-person visits, but that changed in 2020.
At the height of the COVID-19 pandemic, the DEA "temporarily waived the requirement that prescribers meet patients in person before treating them with several classes of drugs," including controlled substances like Adderall and ketamine, reported the Post. The Biden administration has extended the regulatory changes, and a DEA spokesperson told the Post the agency is working on making the changes permanent.
At least eight companies have begun offering ketamine via telehealth since the beginning of the pandemic. Three of the providers combined, including "two of the better-known companies, Nue Life and Mindbloom," have prescribed over 10,000 patients at-home ketamine treatments.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Is it safe to use outside of clinical settings?
While "virtual ketamine startups say they're making the treatment vastly more accessible and improving patients' lives," the Post stated that many psychiatrists "worry that having patients taking it outside a doctor's direct supervision is a step too far, too soon."
"I'm very concerned about treatments that deviate too far from the standard recommendations given by the FDA," Gerard Sanacora, the director of the Yale Depression Research Program who led a team that pioneered ketamine to treat depression, said to the Post. He still believes ketamine "is one of the major advances of psychiatry in the past half-century." Still, health care professionals "have to be very careful to continue to develop this responsibly."
"While many patients have benefited, the rapid growth of remote prescribing and at-home use of various drugs has outpaced the evidence that doing so is safe and effective," Chris Hamby wrote for The New York Times. Hamby interviewed "more than 40 patients who said their access to the drug was expanded through telehealth" and found that some admitted to abusing their prescriptions and hiding adverse side effects from their providers. "The shift away from clinics has led many patients to take the drug more frequently and for longer periods of time," he said, "despite scant research on safety."
Another concern about the safety of mail-order ketamine is that many of the prescriptions are filled by "compounding pharmacies." At-home treatments created a demand for ketamine that was "less potent and easier to take" than the type typically used as anesthesia, Hamby said in the Times. "These specialized companies operate in a murky regulatory space somewhere between a corner drugstore and a pharmaceutical manufacturer." They produce variations of FDA-approved drugs "but do not have to follow the same quality-control rules as drugmakers."
Dr. Michael D. Banov, a psychiatrist in Atlanta, and Dr. Rachel E. Landrum, of Louisiana State University, warn that taking the ketamine unsupervised poses a risk of interactions with other drugs patients might be taking. "There's a risk with combining at-home ketamine with alcohol or drugs of abuse including opiates, amphetamines or benzodiazepines, or with legitimately prescribed medications that could have serious and life-threatening interactions." they wrote in a case study for the Psychiatric Times.
What's next?
The impending end of pandemic-era health measures could spell the end of at-home ketamine treatments, "potentially leaving patients high and dry and the internet upstarts that supply them looking for a contingency plan," according to Bloomberg. The pandemic-era public health emergency is scheduled to end in May, and without an extension, so will access to ketamine without an in-person consultation. The DEA has proposed new guidelines for how telehealth can be used when prescribing Schedule III-V substances. The proposal would allow health care providers to prescribe a 30-day supply of a controlled substance via telehealth but then require an in-person meeting to receive additional prescriptions.
Theara Coleman has worked as a staff writer at The Week since September 2022. She frequently writes about technology, education, literature and general news. She was previously a contributing writer and assistant editor at Honeysuckle Magazine, where she covered racial politics and cannabis industry news.
-
 The Olympic timekeepers keeping the Games on track
The Olympic timekeepers keeping the Games on trackUnder the Radar Swiss watchmaking giant Omega has been at the finish line of every Olympic Games for nearly 100 years
-
 Will increasing tensions with Iran boil over into war?
Will increasing tensions with Iran boil over into war?Today’s Big Question President Donald Trump has recently been threatening the country
-
 Corruption: The spy sheikh and the president
Corruption: The spy sheikh and the presidentFeature Trump is at the center of another scandal
-
 'TikTok brain' may be coming for your kid's attention span
'TikTok brain' may be coming for your kid's attention spanThe Explainer What happens to kids' brains when they binge TikTok's endless stream of bite-sized videos?
-
 The 'girl dinner' TikTok trend has dieticians on edge
The 'girl dinner' TikTok trend has dieticians on edgeSpeed Read Is it a cute and relatable social media fad or a cover for disordered eating?
-
 Understanding the new Covid-19 variant, Eris
Understanding the new Covid-19 variant, ErisSpeed Read The formally named EG.5 is making the rounds, but we don't have to worry just yet
-
 When therapy-speak enters the real world
When therapy-speak enters the real worldSpeed Read Are you "setting boundaries" or avoiding confrontation?
-
 The new push to solve long Covid
The new push to solve long CovidSpeed Read Patients say researchers have been too slow to address the condition
-
 What is cardiac arrest and why does it happen?
What is cardiac arrest and why does it happen?Speed Read The heart condition impacts younger athletes more often than expected
-
 What is medical identity theft and how can you avoid it?
What is medical identity theft and how can you avoid it?The Explainer Scammers can often target medical insurance as part of their grift
-
 The problem with self-diagnosing
The problem with self-diagnosingSpeed Read Teens are turning to social media to diagnose themselves with mental health conditions
