The FDA just approved a 'smart' pill that can track if you're taking your medication

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
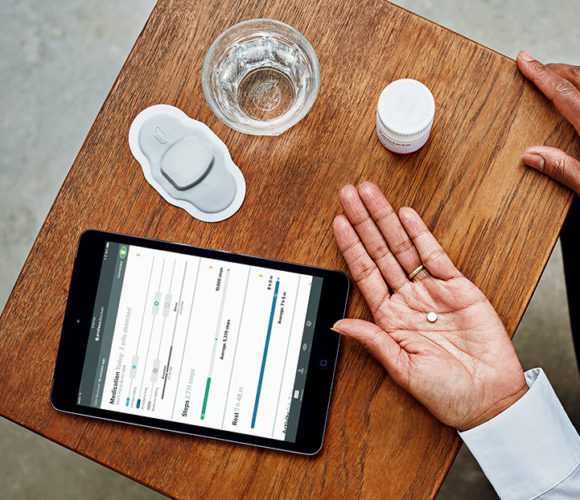
The Food and Drug Administration just approved its first-ever pill containing electronic tracking sensors. The anti-psychotic drug Abilify MyCite, which treats bipolar disorder, schizophrenia, and depressive disorders, uses an electronic signal to record whether or not a patient ingested the pill, Gizmodo reported Tuesday.
When it touches stomach acid, the pill generates an electronic signal, which can be used to send data to medical professionals via a smartphone app and a Bluetooth signal. Sending data requires patients to first sign a consent form allowing their data to be shared with their doctors — as well as up to four selected friends and family members — and also wear a patch on their left rib cage that would transmit the information.
The New York Times notes, however, that patients can block data recipients whenever they choose to. That is potentially problematic, Dr. Jeffrey Lieberman, chairman of psychiatry at Columbia University and New York Presbyterian hospital, told the Times: "There's an irony in it being given to people with mental orders that can include delusions. It's like a biomedical Big Brother."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
While patients prone to paranoia may see malicious intent in electronic tracking of drug ingestion, there are significant benefits to being able to track whether or not patients are taking their medication. Studies have shown that people usually take only half of their prescribed doses when they are self-administering medication, and a 2013 study estimated that people not taking their pills properly costs the U.S. between $100 billion and $289 billion a year and leads to 125,000 deaths annually.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Kelly O'Meara Morales is a staff writer at The Week. He graduated from Sarah Lawrence College and studied Middle Eastern history and nonfiction writing amongst other esoteric subjects. When not compulsively checking Twitter, he writes and records music, subsists on tacos, and watches basketball.
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 What is the endgame in the DHS shutdown?
What is the endgame in the DHS shutdown?Today’s Big Question Democrats want to rein in ICE’s immigration crackdown
-
 ‘Poor time management isn’t just an inconvenience’
‘Poor time management isn’t just an inconvenience’Instant Opinion Opinion, comment and editorials of the day
-
 TikTok secures deal to remain in US
TikTok secures deal to remain in USSpeed Read ByteDance will form a US version of the popular video-sharing platform
-
 Unemployment rate ticks up amid fall job losses
Unemployment rate ticks up amid fall job lossesSpeed Read Data released by the Commerce Department indicates ‘one of the weakest American labor markets in years’
-
 US mints final penny after 232-year run
US mints final penny after 232-year runSpeed Read Production of the one-cent coin has ended
-
 Warner Bros. explores sale amid Paramount bids
Warner Bros. explores sale amid Paramount bidsSpeed Read The media giant, home to HBO and DC Studios, has received interest from multiple buying parties
-
 Gold tops $4K per ounce, signaling financial unease
Gold tops $4K per ounce, signaling financial uneaseSpeed Read Investors are worried about President Donald Trump’s trade war
-
 Electronic Arts to go private in record $55B deal
Electronic Arts to go private in record $55B dealspeed read The video game giant is behind ‘The Sims’ and ‘Madden NFL’
-
 New York court tosses Trump's $500M fraud fine
New York court tosses Trump's $500M fraud fineSpeed Read A divided appeals court threw out a hefty penalty against President Trump for fraudulently inflating his wealth
-
 Trump said to seek government stake in Intel
Trump said to seek government stake in IntelSpeed Read The president and Intel CEO Lip-Bu Tan reportedly discussed the proposal at a recent meeting
