Scientist removed as director of federal agency working on coronavirus vaccine to file whistleblower complaint

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Attorneys for Rick Bright, the federal scientist who once led the department leading coronavirus vaccine development, said on Thursday he is filing a whistleblower complaint, alleging that he was ousted because he did not promote a drug treatment touted by President Trump.
Bright was removed on Tuesday as director of the Biomedical Advanced Research and Development Authority, and given a job at the National Institutes of Health with fewer responsibilities. Bright's lawyers said they will file formal complaints with the inspector general of the Health and Human Services Department and the federal Office of Special Counsel, which will detail "the retaliatory treatment to which he was subjected by HHS political leadership after raising appropriate science-based concerns about White House pressure on treatment and vaccines related to the COVID-19 pandemic."
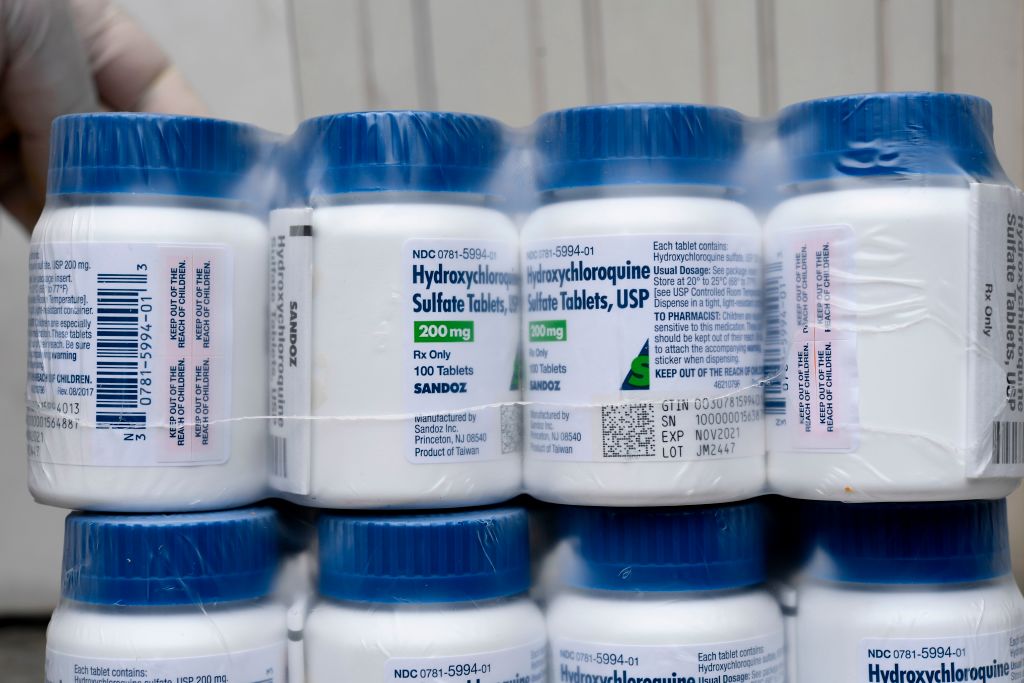
The filings will also "make clear that Dr. Bright was sidelined for one reason only — because he resisted efforts to provide unfettered access to potentially dangerous drugs, including chloroquine, a drug promoted by the administration as a panacea, but which is untested and possibly deadly when used improperly. The facts and concerns raised by Dr. Bright are compelling and well-documented and soon they will be public."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Bright released a statement on Wednesday saying he believes he was removed as director after he made it known he felt "the billions of dollars allocated by Congress to address the COVID-19 pandemic" should be put "into safe and scientifically vetted solutions, and not in drugs, vaccines, and other technologies that lack scientific merit." When asked about this by reporters, Trump said he had never heard of Bright.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Catherine Garcia has worked as a senior writer at The Week since 2014. Her writing and reporting have appeared in Entertainment Weekly, The New York Times, Wirecutter, NBC News and "The Book of Jezebel," among others. She's a graduate of the University of Redlands and the Columbia University Graduate School of Journalism.
-
 Political cartoons for February 16
Political cartoons for February 16Cartoons Monday’s political cartoons include President's Day, a valentine from the Epstein files, and more
-
 Regent Hong Kong: a tranquil haven with a prime waterfront spot
Regent Hong Kong: a tranquil haven with a prime waterfront spotThe Week Recommends The trendy hotel recently underwent an extensive two-year revamp
-
 The problem with diagnosing profound autism
The problem with diagnosing profound autismThe Explainer Experts are reconsidering the idea of autism as a spectrum, which could impact diagnoses and policy making for the condition
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 The new Stratus Covid strain – and why it’s on the rise
The new Stratus Covid strain – and why it’s on the riseThe Explainer ‘No evidence’ new variant is more dangerous or that vaccines won’t work against it, say UK health experts
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
