Moderna vaccine progress a 'positive development' according to former FDA commissioner, but 'there's a lot of work ahead'

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Early data on a coronavirus vaccine candidate is encouraging, but there's still "a lot of work" to be done, the former commissioner of the FDA says.
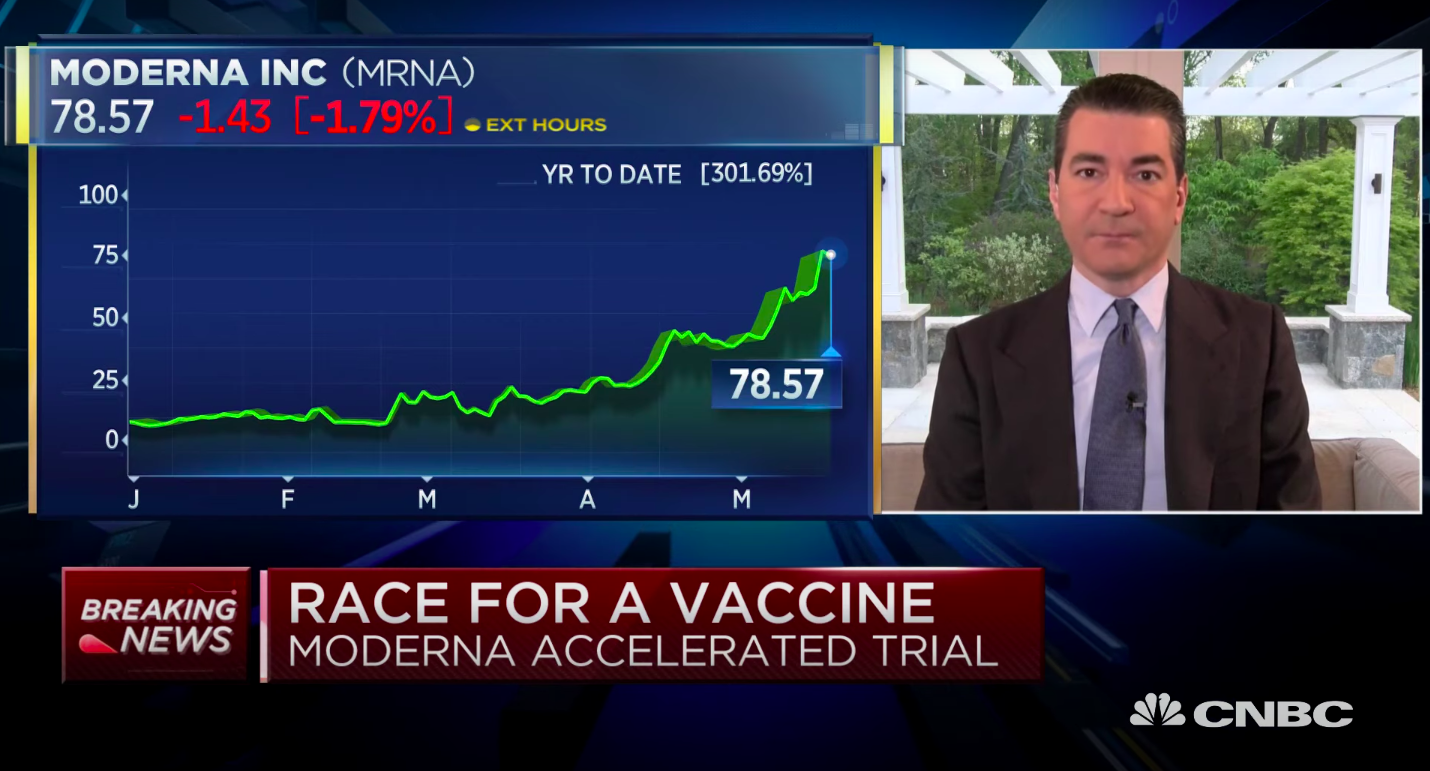
Moderna on Monday announced some "positive" interim data on its potential COVID-19 vaccine, saying that eight patients in a phase one trial developed antibodies at levels similar to those who recovered from the coronavirus. The stock market surged in reaction to the hope that a vaccine could be on the horizon.
Scott Gottlieb, former Food and Drug Administration commissioner, in an interview on CNBC Tuesday agreed this was a "positive development," since it suggests the vaccine "can produce an immune response." He noted, though, that since Moderna only tested the first eight patients of 45 for neutralizing antibodies, "we need to know the data on the other patients, and whether in fact those are neutralizing antibodies, the kinds of antibodies that target the virus and destroy the virus."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"They need to figure out what the right dose is for this vaccine, so they're going to need to do a lot of phase two work before they can get into a phase three trial to determine the optimal dose," Gottlieb said. "We also have to make sure that these vaccines are producing neutralizing antibodies."
Based on early data, it now seems "reasonable" to assume we'll get a vaccine against COVID-19 "at some point," Gottlieb concluded. But when? Though President Trump has touted a potential end-of-year timeline, Gottlieb, who previously said a widely-available vaccine is likely a "2021 event," isn't sure about this incredibly tight schedule.
"The timing is questionable," he said, "whether or not we can have it before the end of the year, or whether or not we'll have to wait until 2021 to have a product we can use more generally." Brendan Morrow
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brendan worked as a culture writer at The Week from 2018 to 2023, covering the entertainment industry, including film reviews, television recaps, awards season, the box office, major movie franchises and Hollywood gossip. He has written about film and television for outlets including Bloody Disgusting, Showbiz Cheat Sheet, Heavy and The Celebrity Cafe.
-
 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 Earth is rapidly approaching a ‘hothouse’ trajectory of warming
Earth is rapidly approaching a ‘hothouse’ trajectory of warmingThe explainer It may become impossible to fix
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
