FDA chief admits he oversold COVID-19 plasma effectiveness


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Scientists were immediately skeptical when Food and Drug Administration chief Stephen Hahn, Health Secretary Alex Azar, and President Trump announced Sunday evening that the FDA had given emergency use approval for plasma from recovered COVID-19 patients, right before the Republican National Convention. And they were baffled when all three men claimed plasma had been shown to reduce deaths by 35 percent, meaning, Hanh said, that 35 out of 100 COVID-19 patients "would have been saved because of the administration of plasma."
That impressive statistic was evidently excavated from a small subsample of a large observational study from the Mayo Clinic, and it doesn't mean what Trump, Azar, and Hahn said it does. Hahn and whoever else came up with the number "appeared to have mixed up absolute risk and relative risk, which are basic concepts in economics and in the presentation of data from clinical trials," The Washington Post notes, explaining:
Essentially, the Trump administration figures had compared one group of patients who got a certain kind of plasma with a group who got a different concentration at a different point in the disease, thus showing the relative difference between those groups. It was not a measure of what happens when some patients get plasma and some don't — the kind of research necessary to send a signal of whether a treatment is truly helping. The FDA also considered data from other studies. [The Washington Post]
After facing criticism from incredulous medical scientists, Hanh acknowledged his error:
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Scientists have reasonable hopes that convalescent plasma, a century-old treatment, will be effective at helping COVID-19 patients recover, at least until a reliable treatment is found or developed. But so far the evidence is "still very low-quality" and "not conclusive, World Health Organization chief scientist Dr. Soumya Swaminathan cautioned Monday.
It isn't really the exaggeration of plasma's benefits that worry medical experts, The New York Times reports. It's that, given how "Trump has appeared to politicize the process of approving treatments and vaccines for the coronavirus," nobody will believe "the scientific judgment of the FDA" when it says a vaccine is safe and effective.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
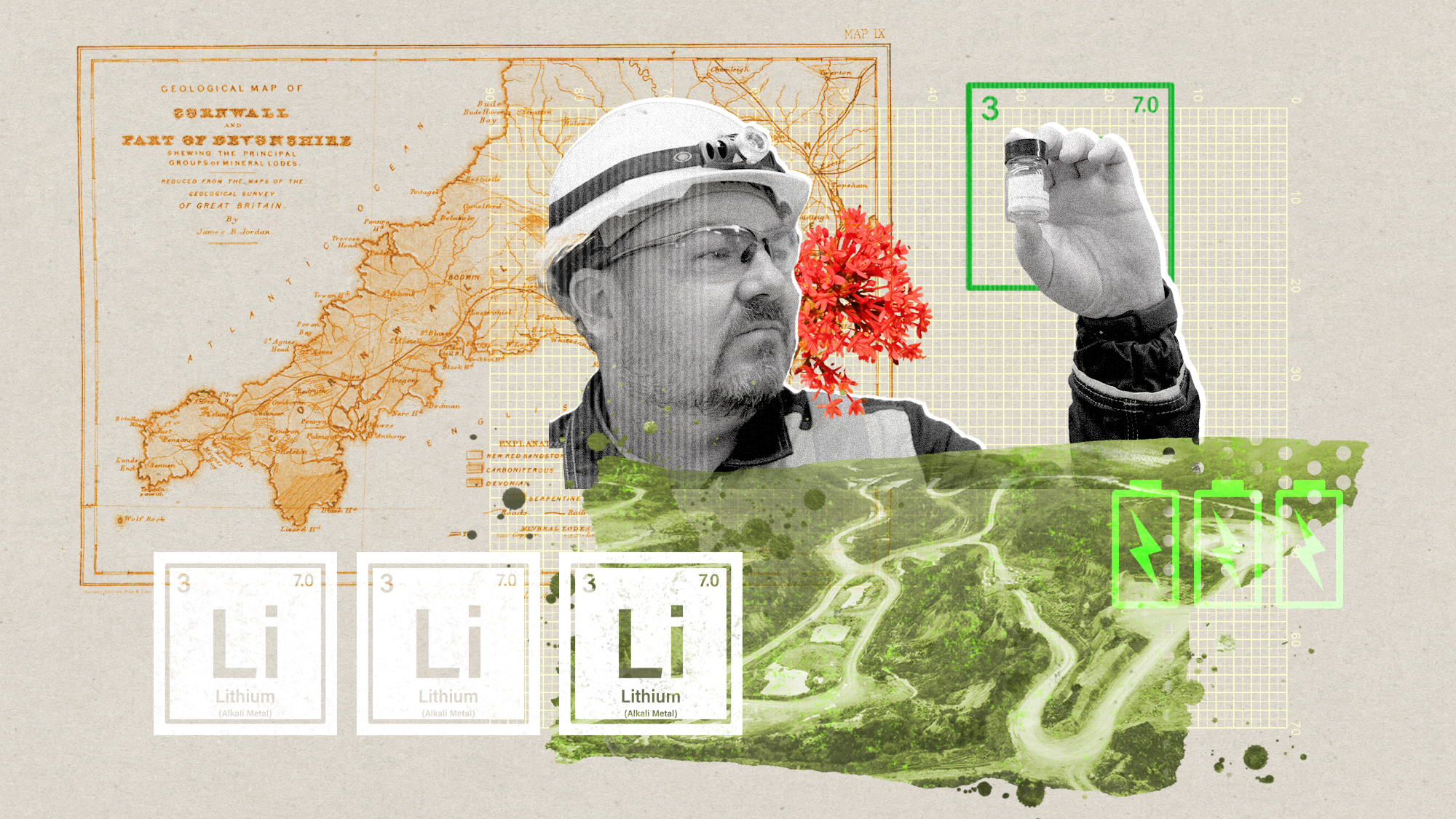 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
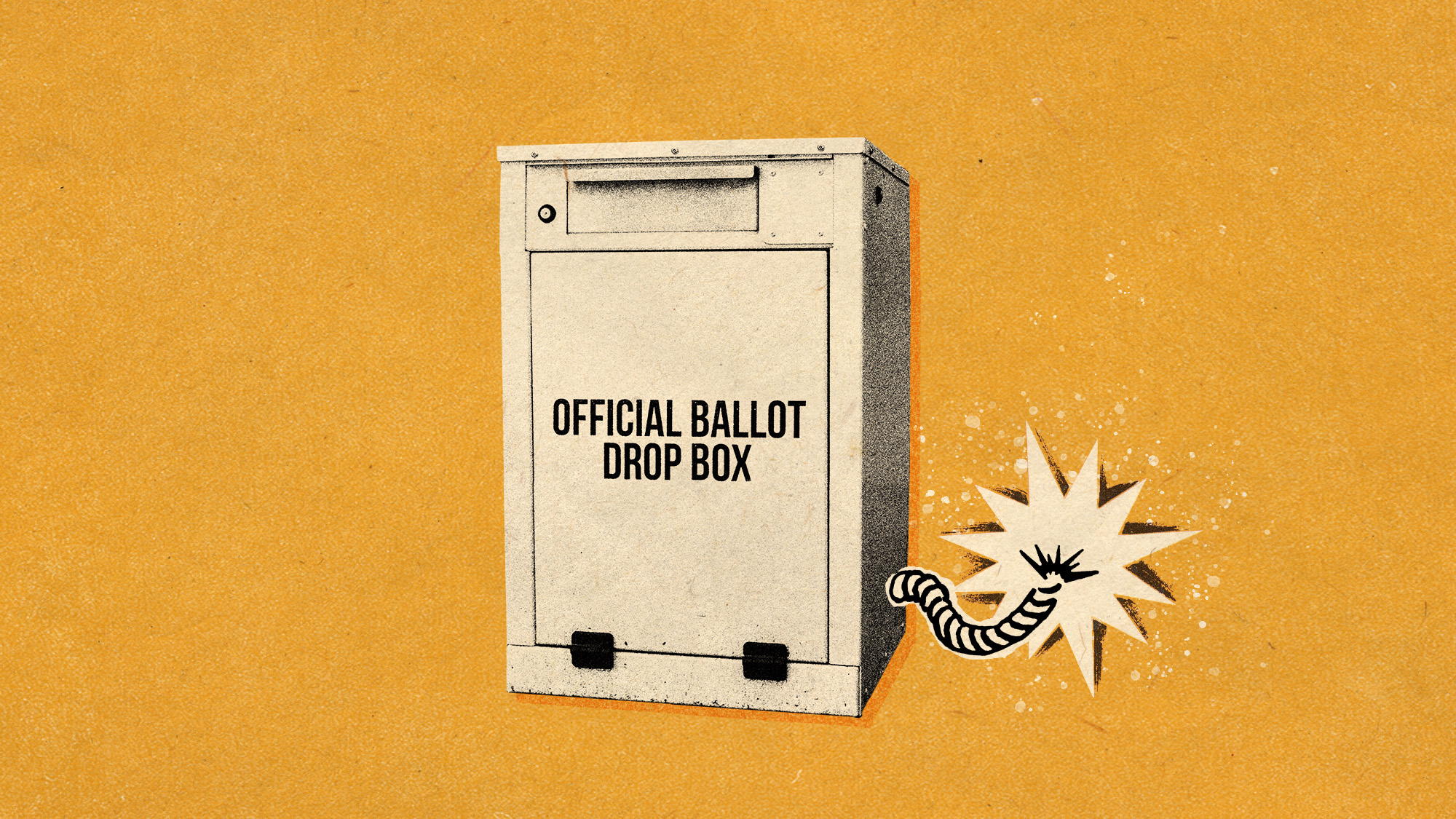 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
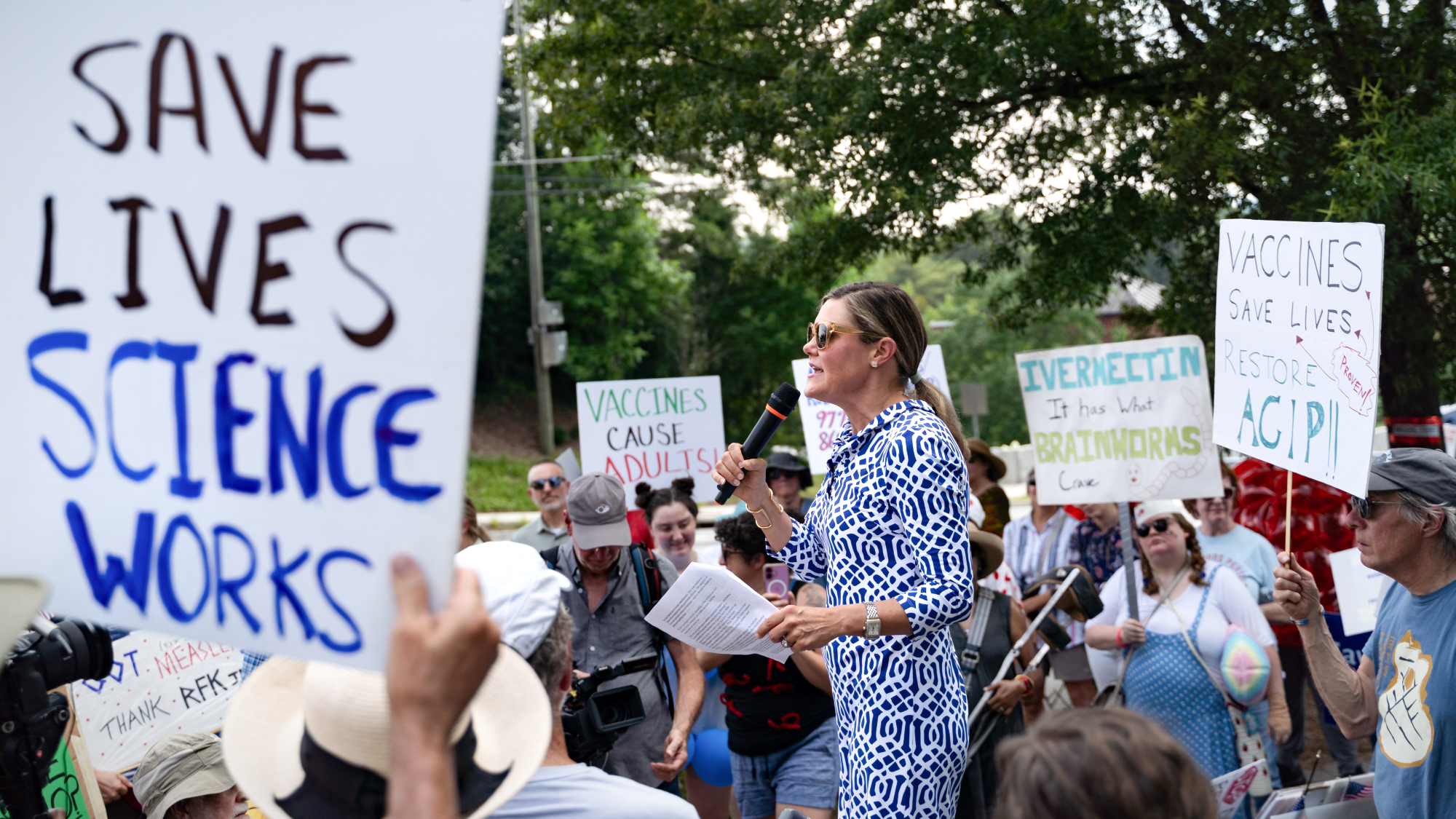 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
