FDA greenlights remdesivir as first and only fully-approved treatment for COVID-19

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
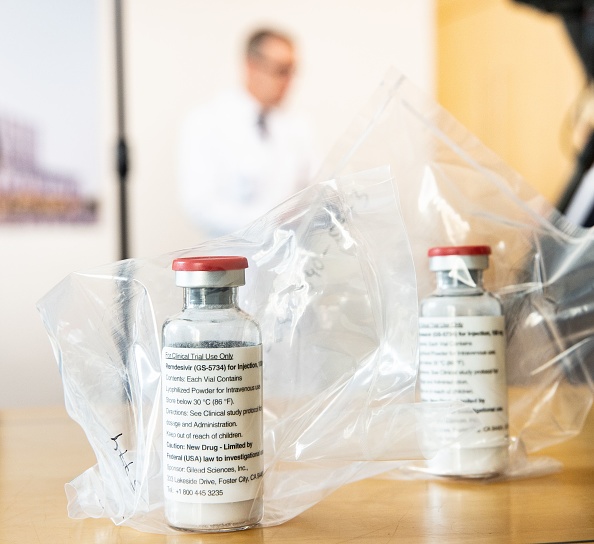
The Food and Drug Administration has given final approval to remdesivir as a treatment for COVID-19, making it the first and only fully-approved treatment in the U.S. for the novel coronavirus, CNBC reports. The drug has been permitted in cases of emergency use authorization since May, and was one of the medications used to treat President Trump when he was hospitalized earlier this month.
Remdesivir (sold under the brand name Veklury) is administered via an IV, and is intended for "the treatment of COVID-19 requiring hospitalization," the drugmaker, Gilead Sciences, said in a statement. While it is approved or authorized for temporary use in around 50 countries, a World Health Organization study earlier this month of some 2,750 patients found that remdesivir had "little or no effect" on death rates.
In a separate study by Gilead Sciences of 1,060 patients, the drug was found to prevent people from "getting sicker" and "from going onto more oxygen support," though the drugmaker likewise "did not find a statistically significant reduction in death rates across the entirety of patients treated in the trial," CNBC writes.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Jeva Lange was the executive editor at TheWeek.com. She formerly served as The Week's deputy editor and culture critic. She is also a contributor to Screen Slate, and her writing has appeared in The New York Daily News, The Awl, Vice, and Gothamist, among other publications. Jeva lives in New York City. Follow her on Twitter.
-
 The 8 best TV shows of the 1960s
The 8 best TV shows of the 1960sThe standout shows of this decade take viewers from outer space to the Wild West
-
 Microdramas are booming
Microdramas are boomingUnder the radar Scroll to watch a whole movie
-
 The Olympic timekeepers keeping the Games on track
The Olympic timekeepers keeping the Games on trackUnder the Radar Swiss watchmaking giant Omega has been at the finish line of every Olympic Games for nearly 100 years
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
