Large 'real-world' study of Pfizer's COVID-19 vaccine confirms efficacy across most age groups

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
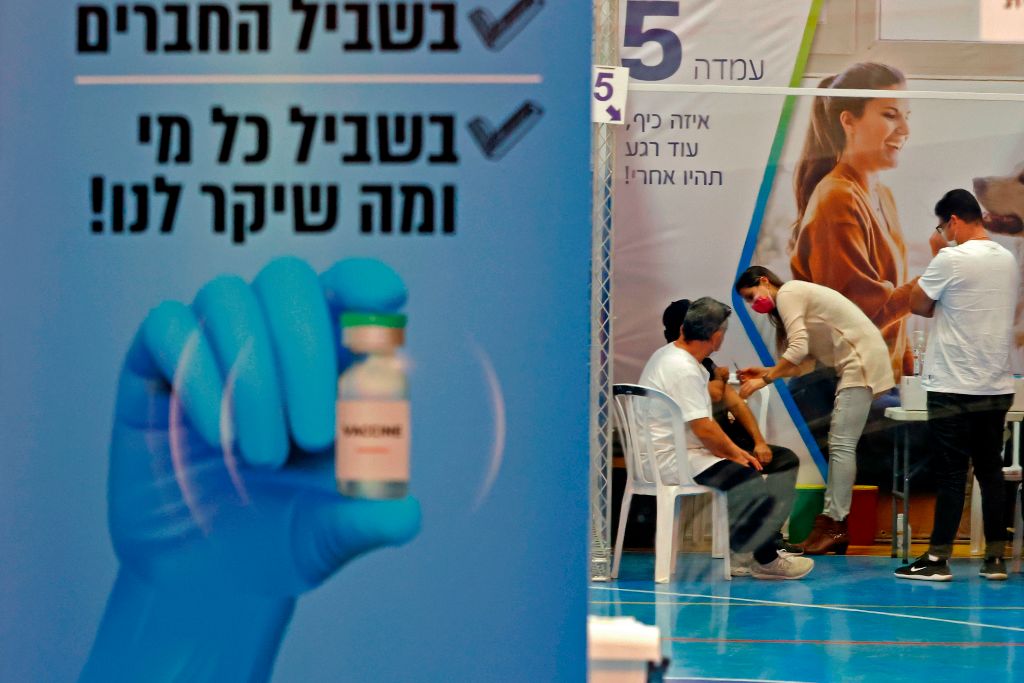
The Pfizer-BioNTech COVID-19 vaccine was basically as effective in a study of 600,000 Israelis as in Pfizer's smaller clinical trials, researchers reported Wednesday in the New England Journal of Medicine. And the new study provided evidence Pfizer was unable to show, due to limited data in its trials, that the vaccine is equally effective in people 70 and over as in younger people.
The publisher results provide "scientifically validated real-world evidence of the effectiveness of the vaccine," said Ran Balicer, one of the study's chief authors and chief innovation officer at Clalit, Israel's largest health care provider.
The study compared 600,000 Israelis who had received the vaccine against 600,000 demographically similar Israelis who were not vaccinated. The vaccinated population was 57 percent less likely to develop any COVID-19 symptoms in the two to three weeks after getting the first dose and 94 percent less likely after getting the second shot. Those fully inoculated were 92 percent less likely to develop severe COVID-19 symptoms. One dose was 72 percent effective at preventing death.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"This is immensely reassuring," said the Mayo Clinic's Dr. Gregory Poland, who is not involved in the study, "better than I would have guessed." The Clalit Research Institute, Israel's Ben-Gurion University of the Negev, and Harvard University led the research.
Israel agreed to give Pfizer access to its data in exchange for extra vaccine supply, and it has now given more than half its population a first dose and fully inoculated about a third of the country. Its data is especially useful because Israel has now opened its vaccination campaign to everyone older than 16. This has also allowed Israel to experiment with the next stages of the pandemic. On Saturday, Israel began issuing special passports that allow vaccinated people access to concerts, gyms, hotels, and other public spaces.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
 Quiz of The Week: 14 – 20 February
Quiz of The Week: 14 – 20 FebruaryQuiz Have you been paying attention to The Week’s news?
-
 The Week Unwrapped: Do the Freemasons have too much sway in the police force?
The Week Unwrapped: Do the Freemasons have too much sway in the police force?Podcast Plus, what does the growing popularity of prediction markets mean for the future? And why are UK film and TV workers struggling?
-
 Properties of the week: pretty thatched cottages
Properties of the week: pretty thatched cottagesThe Week Recommends Featuring homes in West Sussex, Dorset and Suffolk
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
