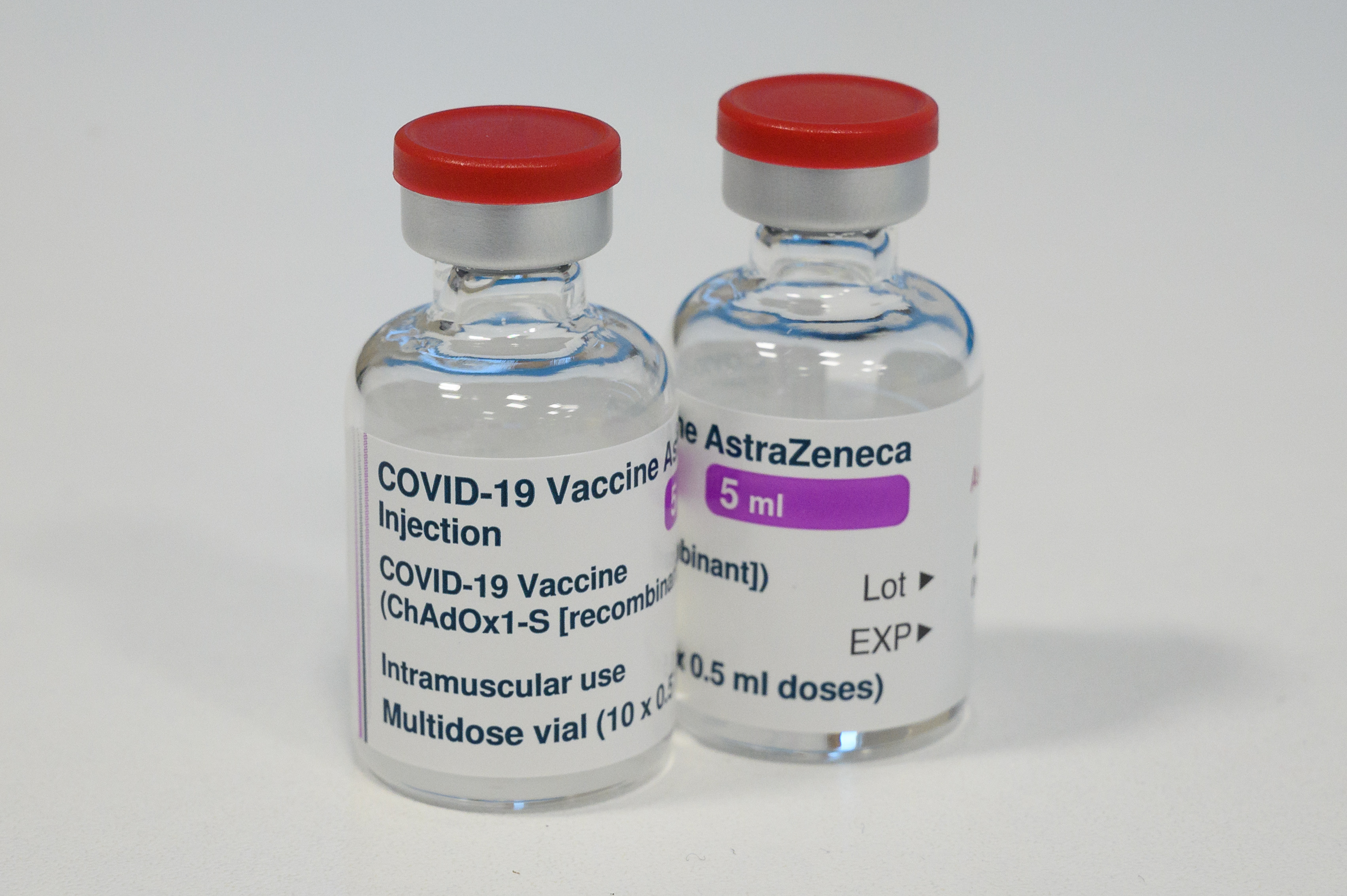
AstraZeneca says revised data shows COVID-19 vaccine 76 percent effective

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
AstraZeneca said in a press release late Wednesday that a new analysis of its large U.S. clinical trial found its beleaguered COVID-19 vaccine to be 76 percent effective at preventing symptomatic COVID-19 and 100 percent effective at averting severe illness and hospitalization. The new findings, which include 49 more COVID-19 cases from March, lowered the efficacy rate slightly from the 79 percent reported Monday.
The AstraZeneca vaccine, developed by Oxford University researchers, has suffered an unusually rocky rollout. In the most recent drama, the independent monitoring board overusing AstraZeneca's 32,000-volunteer U.S. trial pushed the Anglo-Swedish company to update its analysis based on more recent data, including the National Institute of Allergy and Infectious Diseases in its letter. The NIAID then said in a public statement that AstraZeneca's "outdated information" may have given an "incomplete view of the efficacy data."
"This is really what you call an unforced error, because the fact is this is very likely a very good vaccine," NIAID Director Dr. Anthony Fauci said on Tuesday's Good Morning America.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The new data includes 190 COVID-19 cases that occurred during the study and 14 possible cases that are still being analyzed, meaning the efficacy rate could still change slightly. Eight people, all of whom received placebos instead of the vaccine, became severely ill. The new data bumped the efficacy rate among vaccine recipients 65 and older up to 85 percent, from 80 percent. AstraZeneca said it will submit its final analysis for peer review in a journal.
"Based on a statistical measure called confidence intervals given in the press release, the end result on overall efficacy could be anywhere between 68 percent and 82 percent — figures that would more than pass the Food and Drug Administration's criteria for an emergency use authorization," Stat News reports. The FDA will decide based on an independent analysis of AstraZeneca's raw data.
Eric Topol, director of the Scripps Research Translational Institute, told Stat the revised results are "better than expected," given the public fight with the monitoring board and NIAID, and he's "relieved since the world needs the vaccine badly." The "3 percentage point difference in efficacy isn't much, but the public relations damage to the vaccine brought on by the last few days may be hard to undo," Caitlin Owens notes at Axios. Several researchers told Stat that after all the twist and turns with AstraZeneca, they will withhold judgment until more results are available.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
 What to know before filing your own taxes for the first time
What to know before filing your own taxes for the first timethe explainer Tackle this financial milestone with confidence
-
 The biggest box office flops of the 21st century
The biggest box office flops of the 21st centuryin depth Unnecessary remakes and turgid, expensive CGI-fests highlight this list of these most notorious box-office losers
-
 What are the best investments for beginners?
What are the best investments for beginners?The Explainer Stocks and ETFs and bonds, oh my
-
 TikTok secures deal to remain in US
TikTok secures deal to remain in USSpeed Read ByteDance will form a US version of the popular video-sharing platform
-
 Unemployment rate ticks up amid fall job losses
Unemployment rate ticks up amid fall job lossesSpeed Read Data released by the Commerce Department indicates ‘one of the weakest American labor markets in years’
-
 US mints final penny after 232-year run
US mints final penny after 232-year runSpeed Read Production of the one-cent coin has ended
-
 Warner Bros. explores sale amid Paramount bids
Warner Bros. explores sale amid Paramount bidsSpeed Read The media giant, home to HBO and DC Studios, has received interest from multiple buying parties
-
 Gold tops $4K per ounce, signaling financial unease
Gold tops $4K per ounce, signaling financial uneaseSpeed Read Investors are worried about President Donald Trump’s trade war
-
 Electronic Arts to go private in record $55B deal
Electronic Arts to go private in record $55B dealspeed read The video game giant is behind ‘The Sims’ and ‘Madden NFL’
-
 New York court tosses Trump's $500M fraud fine
New York court tosses Trump's $500M fraud fineSpeed Read A divided appeals court threw out a hefty penalty against President Trump for fraudulently inflating his wealth
-
 Trump said to seek government stake in Intel
Trump said to seek government stake in IntelSpeed Read The president and Intel CEO Lip-Bu Tan reportedly discussed the proposal at a recent meeting
