White House may need to scale back booster shot plan

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
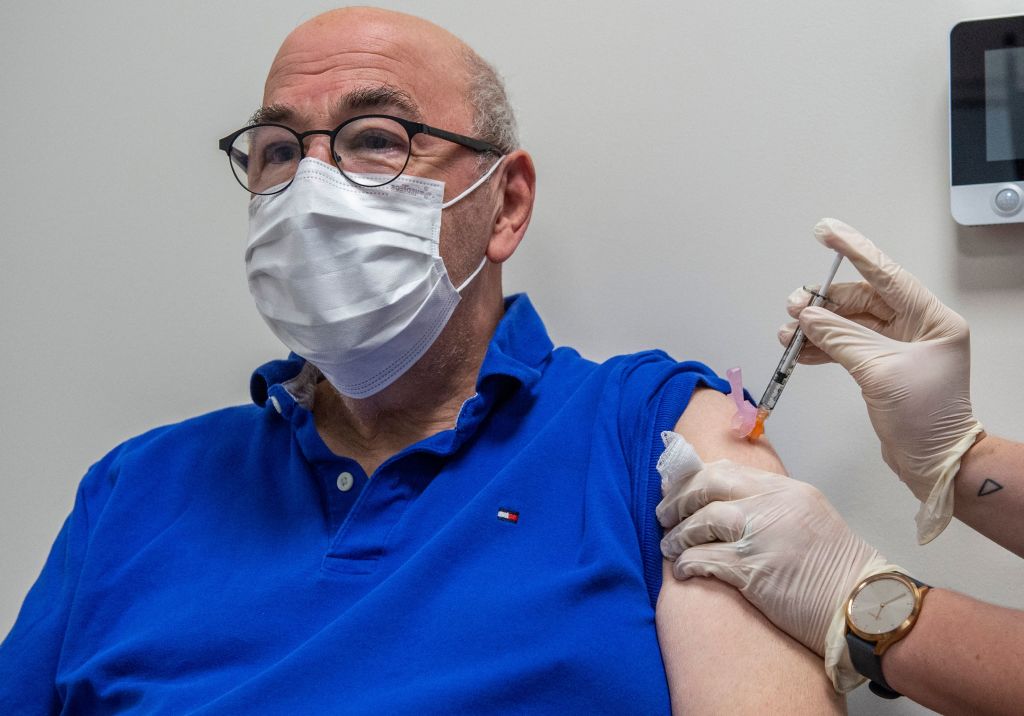
The Biden administration last month unveiled plans to start offering COVID-19 booster shots to all Americans beginning on Sept. 20. But officials are reportedly now weighing whether this plan will have to be scaled back.
Top health officials are urging the White House to scale back the COVID-19 booster shot plan because more time is needed to review the data, The New York Times reported. CNN also reported that the Biden administration is discussing whether to scale the plan back, citing an official as saying the FDA doesn't currently have enough data to recommend a third dose of the Moderna vaccine. "The officials warned that more time may be needed before enough data is in to recommend boosters for all adults," CNN writes. Data that Moderna recently submitted was "found inadequate and needs strengthening," an official said, NBC News reports.
When announcing the booster rollout plan last month, top health officials said the administration was "prepared to offer booster shots for all Americans beginning the week of September 20" subject to FDA approval. The booster shots, the officials said, would be offered starting eight months after an individual received their second dose, though The Wall Street Journal later reported that regulators would likely approve the shots after six months.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
A White House spokesperson told the Times that "we always said we would follow the science, and this is all part of a process that is now underway." The spokesperson added that when the booster shots received "full review and approval" from the FDA, the administration "will be ready to implement the plan our nation's top doctors developed so that we are staying ahead of this virus."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brendan worked as a culture writer at The Week from 2018 to 2023, covering the entertainment industry, including film reviews, television recaps, awards season, the box office, major movie franchises and Hollywood gossip. He has written about film and television for outlets including Bloody Disgusting, Showbiz Cheat Sheet, Heavy and The Celebrity Cafe.
-
 6 exquisite homes with vast acreage
6 exquisite homes with vast acreageFeature Featuring an off-the-grid contemporary home in New Mexico and lakefront farmhouse in Massachusetts
-
 Film reviews: ‘Wuthering Heights,’ ‘Good Luck, Have Fun, Don’t Die,’ and ‘Sirat’
Film reviews: ‘Wuthering Heights,’ ‘Good Luck, Have Fun, Don’t Die,’ and ‘Sirat’Feature An inconvenient love torments a would-be couple, a gonzo time traveler seeks to save humanity from AI, and a father’s desperate search goes deeply sideways
-
 Political cartoons for February 16
Political cartoons for February 16Cartoons Monday’s political cartoons include President's Day, a valentine from the Epstein files, and more
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 The new Stratus Covid strain – and why it’s on the rise
The new Stratus Covid strain – and why it’s on the riseThe Explainer ‘No evidence’ new variant is more dangerous or that vaccines won’t work against it, say UK health experts
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
