FDA restricts Johnson & Johnson COVID vaccine due to blood clot risk

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
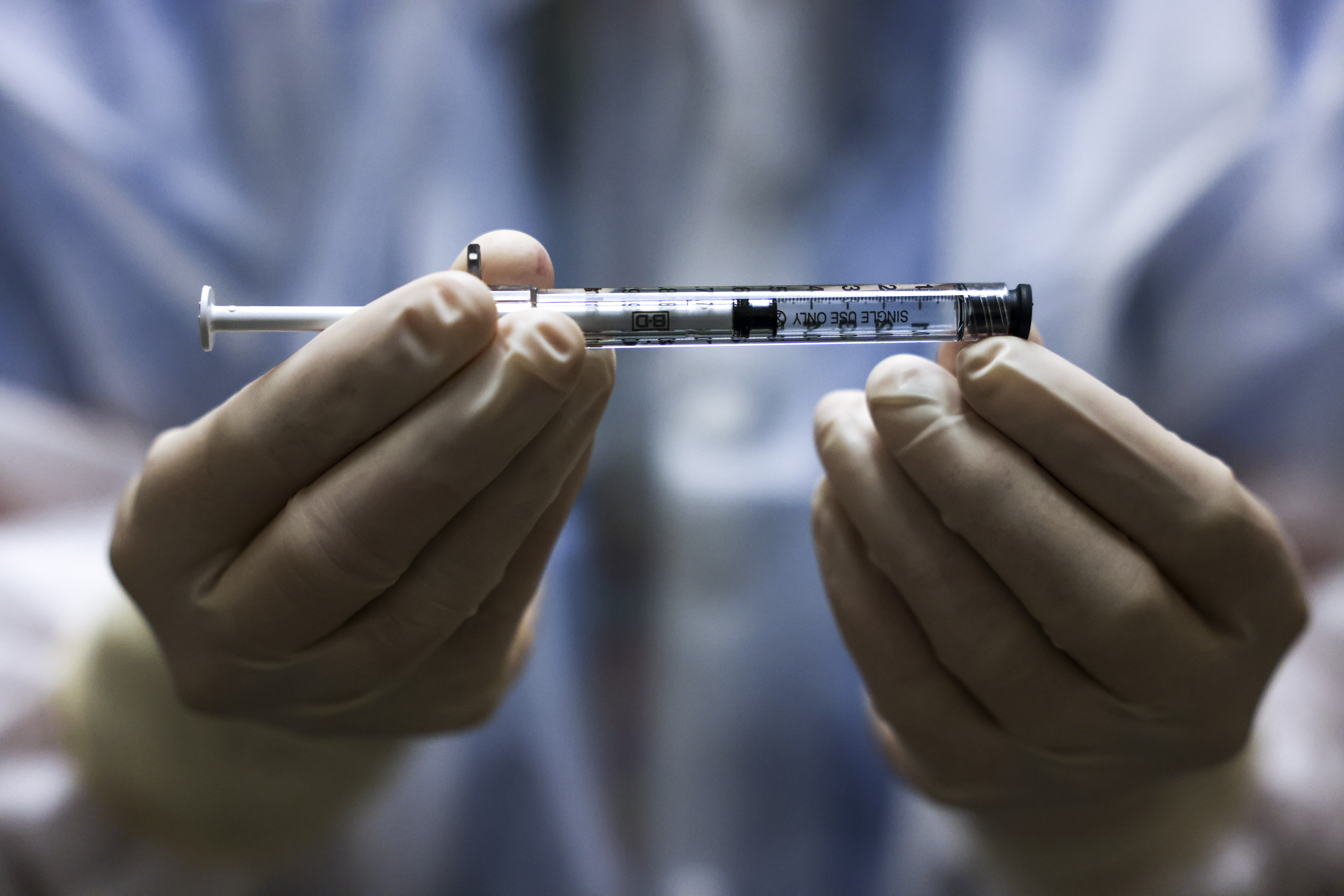
The Food and Drug Administration on Thursday limited who can receive the Johnson & Johnson COVID-19 vaccine, citing concerns over a rare and serious blood clotting condition.
The vaccine is under an emergency use authorization, for adults 18 and older, and the FDA said in a statement that it should now only be given to people who can't or won't get any other vaccine. The agency said it came to this determination after conducting "an updated analysis, evaluation, and investigation" of cases of thrombosis with thrombocytopenia syndrome (TTS) reported after receiving the vaccine. TTS can cause dangerous blood clots.
The FDA said the benefits of the Johnson & Johnson vaccine still outweigh the risks for those who have had a severe allergic reaction to mRNA COVID-19 vaccines, those with limited access to mRNA vaccines, and those who are concerned about mRNA vaccines and will only get a Johnson & Johnson vaccine.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
More than 18.7 million doses of the Johnson & Johnson vaccine have been administered in the United States, the Centers for Disease Control and Prevention said Thursday. TTS cases usually begin one or two weeks after vaccination, CNN reports, with symptoms including shortness of breath, leg swelling, and chest pain. About three cases have been reported for every 1 million vaccine doses administered, with women between 30 and 49 years of age having the highest rate of cases. Read more at CNN.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Catherine Garcia has worked as a senior writer at The Week since 2014. Her writing and reporting have appeared in Entertainment Weekly, The New York Times, Wirecutter, NBC News and "The Book of Jezebel," among others. She's a graduate of the University of Redlands and the Columbia University Graduate School of Journalism.
-
 Trump’s EPA kills legal basis for federal climate policy
Trump’s EPA kills legal basis for federal climate policySpeed Read The government’s authority to regulate several planet-warming pollutants has been repealed
-
 Political cartoons for February 13
Political cartoons for February 13Cartoons Friday's political cartoons include rank hypocrisy, name-dropping Trump, and EPA repeals
-
 Palantir's growing influence in the British state
Palantir's growing influence in the British stateThe Explainer Despite winning a £240m MoD contract, the tech company’s links to Peter Mandelson and the UK’s over-reliance on US tech have caused widespread concern
-
 Blue Origin launches Mars probes in NASA debut
Blue Origin launches Mars probes in NASA debutSpeed Read The New Glenn rocket is carrying small twin spacecraft toward Mars as part of NASA’s Escapade mission
-
 Dinosaurs were thriving before asteroid, study finds
Dinosaurs were thriving before asteroid, study findsSpeed Read The dinosaurs would not have gone extinct if not for the asteroid
-
 SpaceX breaks Starship losing streak in 10th test
SpaceX breaks Starship losing streak in 10th testspeed read The Starship rocket's test flight was largely successful, deploying eight dummy satellites during its hour in space
-
 Rabbits with 'horns' sighted across Colorado
Rabbits with 'horns' sighted across Coloradospeed read These creatures are infected with the 'mostly harmless' Shope papilloma virus
-
 Lithium shows promise in Alzheimer's study
Lithium shows promise in Alzheimer's studySpeed Read Potential new treatments could use small amounts of the common metal
-
 Scientists discover cause of massive sea star die-off
Scientists discover cause of massive sea star die-offSpeed Read A bacteria related to cholera has been found responsible for the deaths of more than 5 billion sea stars
-
 'Thriving' ecosystem found 30,000 feet undersea
'Thriving' ecosystem found 30,000 feet underseaSpeed Read Researchers discovered communities of creatures living in frigid, pitch-black waters under high pressure
-
 New York plans first nuclear plant in 36 years
New York plans first nuclear plant in 36 yearsSpeed Read The plant, to be constructed somewhere in upstate New York, will produce enough energy to power a million homes
