FDA authorizes COVID-19 Omicron boosters for kids as young as 5

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The U.S. Food and Drug Administration on Wednesday authorized COVID-19 Omicron booster shots for children as young as five, taking an additional step to help kids stay protected against the virus.
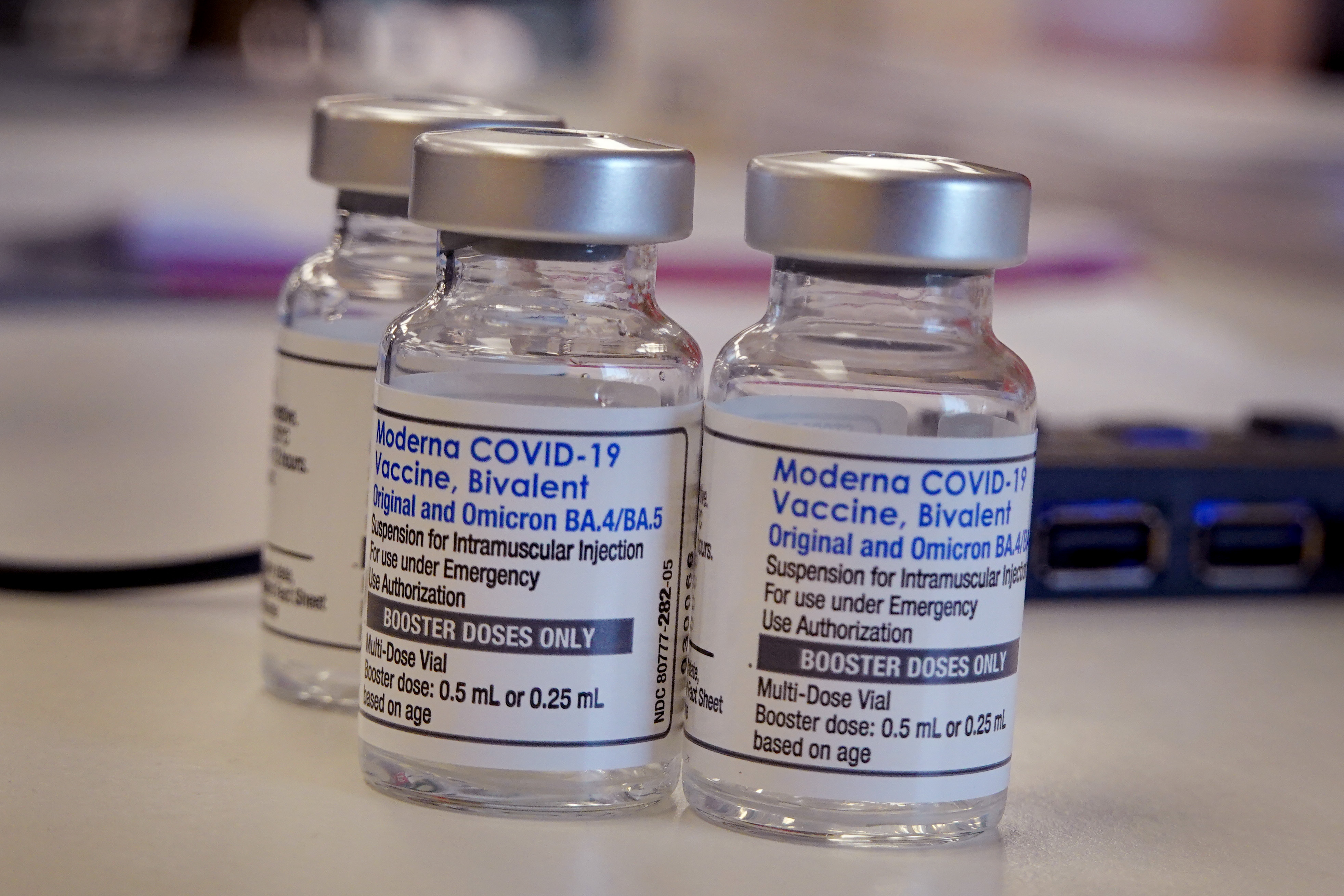
In a statement, the FDA said it had cleared the updated booster from Pfizer and its partner BioNTech for children ages five to 11. The agency also authorized the updated booster from Moderna for children ages six to 17.
Wednesday's news marks a continuing update on the available boosters for children, as the FDA had previously cleared the Pfizer booster for people 12 and over, and the Moderna booster for people 18 and older, per The Wall Street Journal. Children ages five to 11 could have access to the Omicron boosters "in the coming days" if the shots soon receive official clearance from the U.S. Centers for Disease Control and Prevention, the Journal added.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The FDA has continued to push vaccines for kids, particularly as winter approaches. "Since children have gone back to school in person and people are resuming pre-pandemic behaviors and activities, there is the potential for increased risk of exposure to the virus that causes COVID-19," Dr. Peter Marks told the FDA. "Vaccination remains the most effective measure to prevent the severe consequences of COVID-19."
Even as the effort to vaccinate children continues, data from the American Academy of Pediatrics cited by the Journal says that just 31 percent of kids ages five to 11 have completed their primary vaccine series.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Justin Klawans has worked as a staff writer at The Week since 2022. He began his career covering local news before joining Newsweek as a breaking news reporter, where he wrote about politics, national and global affairs, business, crime, sports, film, television and other news. Justin has also freelanced for outlets including Collider and United Press International.
