Moderna coronavirus vaccine candidate receives fast-track designation from the FDA


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The U.S. Food and Drug Administration has given a "fast track" designation to a COVID-19 vaccine candidate in development from Moderna Therapeutics.
Moderna announced on Tuesday that its vaccine candidate has received fast track approval from the FDA, Time reports, with its chief medical officer, Dr. Tal Zaks, saying this "underscores the urgent need for a vaccine against the novel coronavirus." Moderna President Dr. Stephen Hoge also told Time this is "validation that the FDA believes this is a very credible exercise."
The fast track designation, the FDA explains, helps "expedite the review" of the vaccine's development process, with "early and frequent communication between the FDA" and the company. Cutting through some of the red tape will help the vaccine trials accelerate alongside other candidates; health experts like Dr. Anthony Fauci say we likely need more than one vaccine to effectively fight COVID-19 and meet demand.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Last week, Moderna announced it received clearance from the FDA to proceed with phase two testing of this mRNA vaccine candidate, planning for a phase three study in early summer. Modern Chief Executive Officer Stéphane Bancel described the "imminent" start of phase two as a "crucial step forward," and Moderna says its phase two study with 600 participants is "expected to begin shortly." According to CNBC, "If all goes well, its vaccine could be in production as early as July."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brendan worked as a culture writer at The Week from 2018 to 2023, covering the entertainment industry, including film reviews, television recaps, awards season, the box office, major movie franchises and Hollywood gossip. He has written about film and television for outlets including Bloody Disgusting, Showbiz Cheat Sheet, Heavy and The Celebrity Cafe.
-
 The environmental cost of GLP-1s
The environmental cost of GLP-1sThe explainer Producing the drugs is a dirty process
-
 Greenland’s capital becomes ground zero for the country’s diplomatic straits
Greenland’s capital becomes ground zero for the country’s diplomatic straitsIN THE SPOTLIGHT A flurry of new consular activity in Nuuk shows how important Greenland has become to Europeans’ anxiety about American imperialism
-
 ‘This is something that happens all too often’
‘This is something that happens all too often’Instant Opinion Opinion, comment and editorials of the day
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
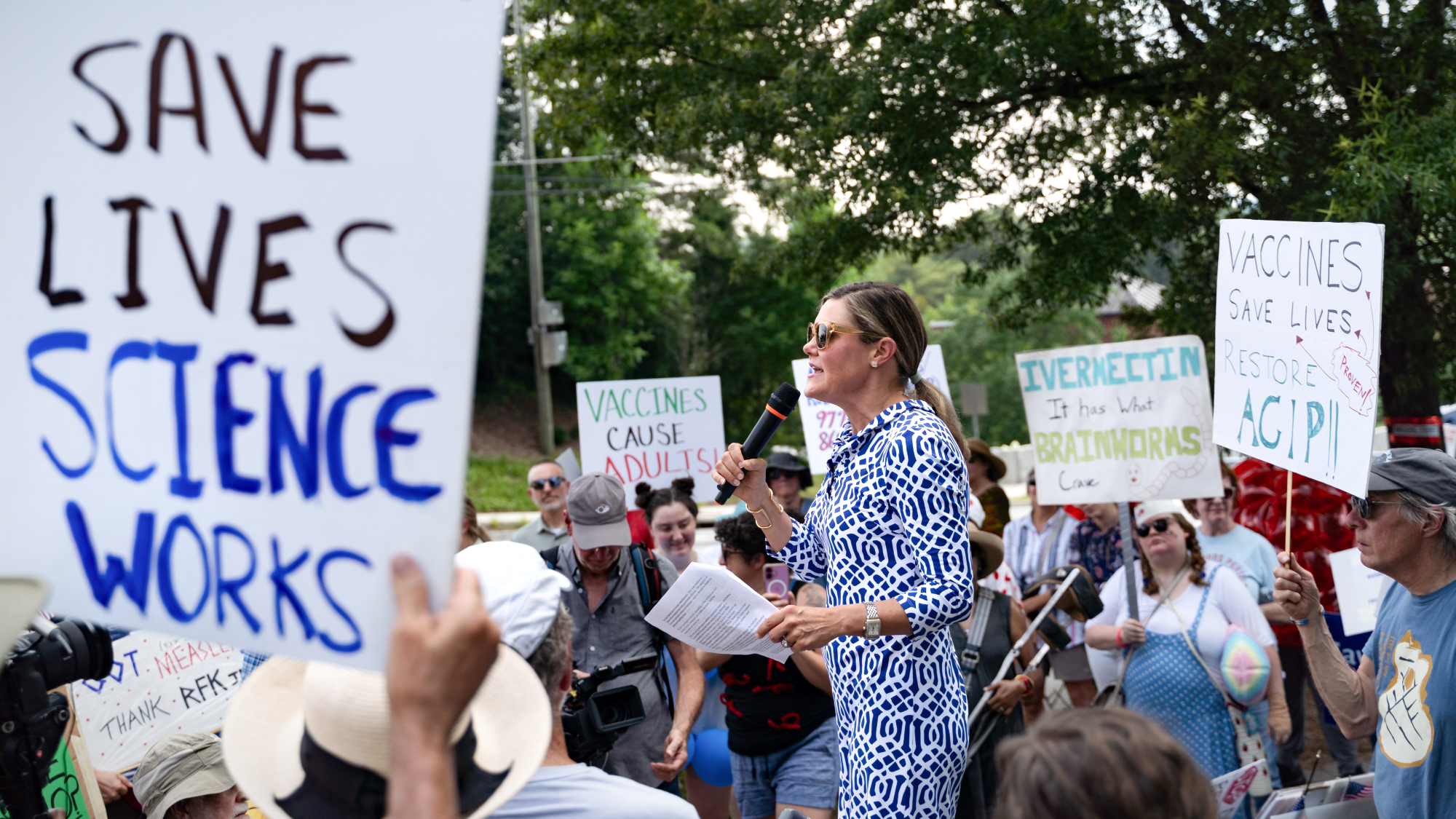 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
