WHO temporarily pauses hydroxychloroquine study, citing safety concerns

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
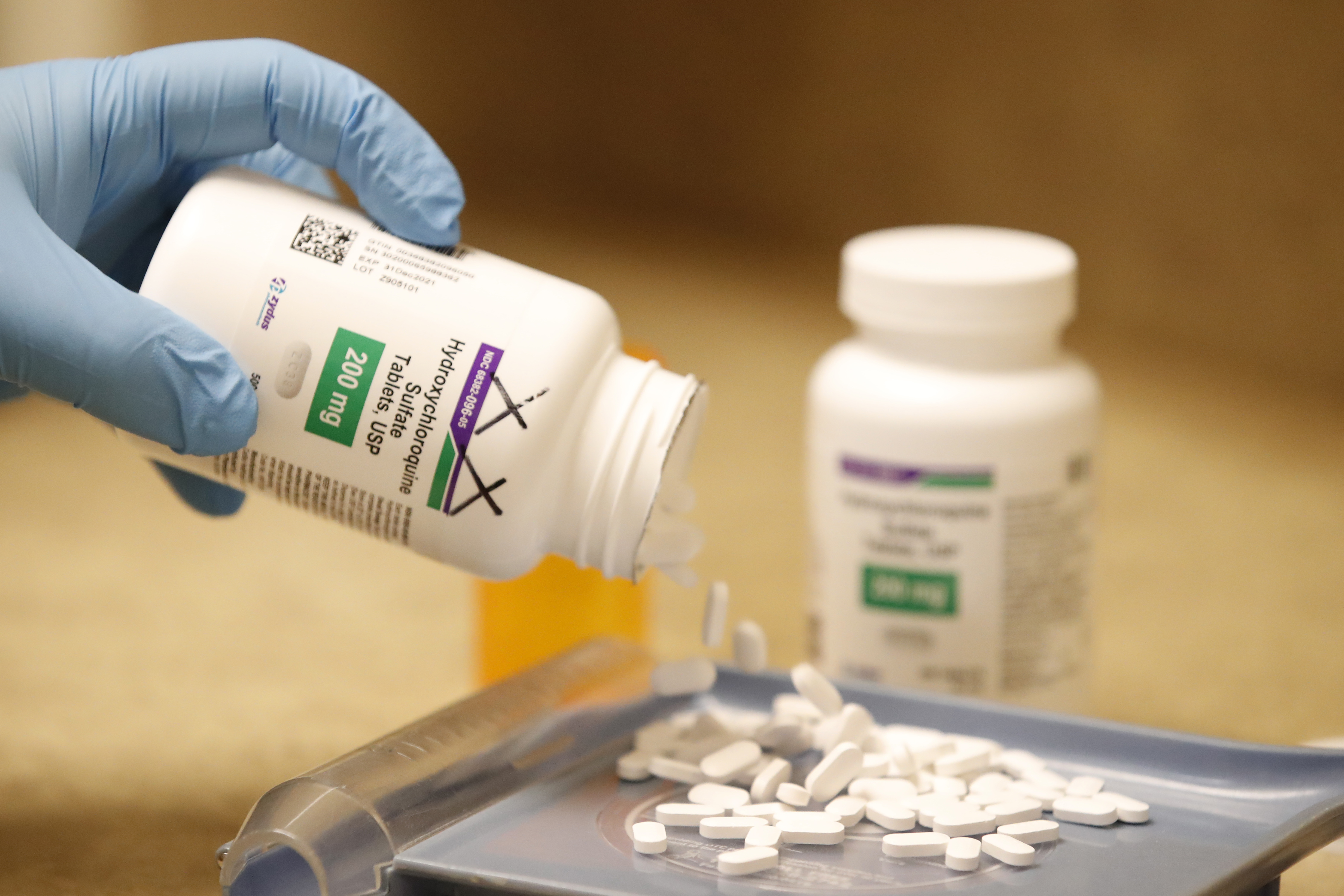
The World Health Organization is temporarily halting a study on hydroxychloroquine and chloroquine as potential COVID-19 treatments due to safety concerns.
WHO Director-General Tedros Adhanom Ghebreyesus announced the decision on Monday, after a study published in The Lancet medical journal suggested COVID-19 patients treated with hydroxychloroquine and chloroquine were more likely to die, CNN reports. The drugs were being reviewed as part of the WHO's Solidarity Trial — a coronavirus research effort involving more than 400 hospitals in 35 countries.
President Donald Trump last week revealed he takes hydroxychloroquine as a preventative measure against COVID-19, despite there being no evidence of its effectiveness and the FDA cautioning against the drug due to serious side effects including abnormal heart rhythms. Meanwhile Brazilian President Jair Bolsonaro is touting the other drug halted in the study, chloroquine.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The Data Safety Monitoring Board will review data about the drugs to assess whether they should continue to be used in the trial, though other arms of the trial will carry on, per CNN.
Tedros noted the halt concerns hydroxychloroquine and chloroquine as they pertain to COVID-19. "I wish to reiterate that these drugs are accepted as generally safe for use in patients with autoimmune diseases or malaria," he said.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Taylor Watson is audience engagement editor for TheWeek.com and a former editorial assistant. She graduated from Syracuse University, with a major in magazine journalism and minors in food studies and nutrition. Taylor has previously written for Runner's World, Vice, and more.
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 What is the endgame in the DHS shutdown?
What is the endgame in the DHS shutdown?Today’s Big Question Democrats want to rein in ICE’s immigration crackdown
-
 ‘Poor time management isn’t just an inconvenience’
‘Poor time management isn’t just an inconvenience’Instant Opinion Opinion, comment and editorials of the day
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
