Johnson & Johnson's early vaccine trial results show most participants developed strong immune response


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Johnson & Johnson announced the start of phase three of its coronavirus vaccine trial this week, citing "positive interim results" from earlier stages of its study. Those were published Friday, and they were indeed promising.
The pharmaceutical giant reported that 99 percent of the participants between the ages of 18 and 55 in early-to-mid stage clinical trials developed neutralizing antibodies against the novel virus. The analysis also found that most of the side effects associated with the vaccine were mild and resolved within a matter of days.
It wasn't clear, however, whether participants over 65 were well-protected since immune response results were available for only 15 people in that demographic. Additionally, Reuters reports, the rate of adverse reactions — like fatigue and muscle aches — to the vaccine in that age group was just 36 percent, far lower than those seen in 64 percent of the younger participants. That might sound like good news, but it actually suggests the immune response in older people may be weaker.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
One of the key aspects of Johnson & Johnson's trial is that just a single dose produced a strong immune response in participants. Other companies developing vaccines like Moderna and Pfizer are using a two-dose approach. If Johnson & Johnson's phase three trial, in which 60,000 volunteers will enroll across three continents, eventually proves the single dose is safe and effective, it could simplify distribution of the vaccine. Read more at Reuters and CNN.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Tim is a staff writer at The Week and has contributed to Bedford and Bowery and The New York Transatlantic. He is a graduate of Occidental College and NYU's journalism school. Tim enjoys writing about baseball, Europe, and extinct megafauna. He lives in New York City.
-
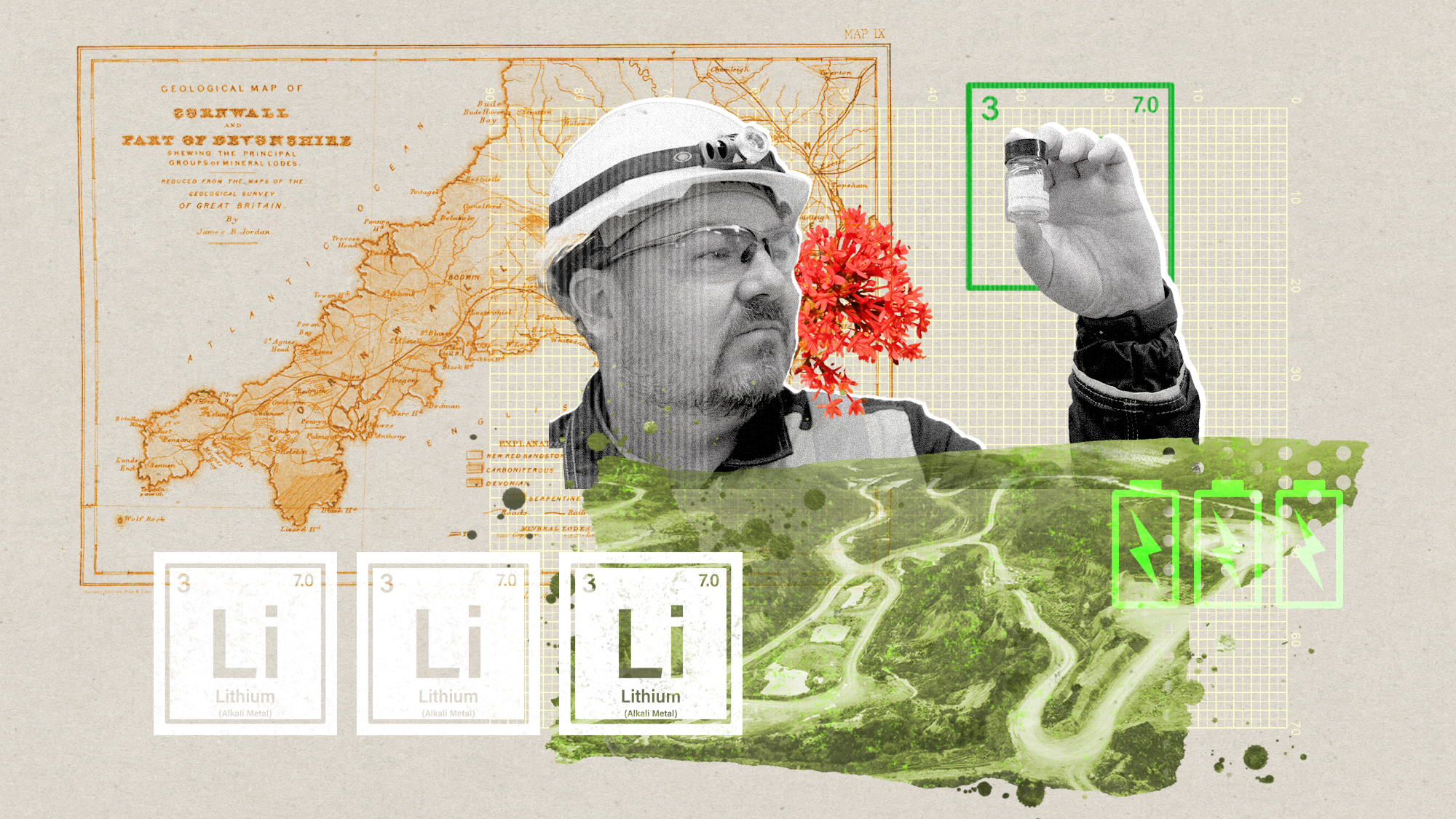 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 ‘One Battle After Another’ wins Critics Choice honors
‘One Battle After Another’ wins Critics Choice honorsSpeed Read Paul Thomas Anderson’s latest film, which stars Leonardo DiCaprio, won best picture at the 31st Critics Choice Awards
-
 Son arrested over killing of Rob and Michele Reiner
Son arrested over killing of Rob and Michele ReinerSpeed Read Nick, the 32-year-old son of Hollywood director Rob Reiner, has been booked for the murder of his parents
-
 Rob Reiner, wife dead in ‘apparent homicide’
Rob Reiner, wife dead in ‘apparent homicide’speed read The Reiners, found in their Los Angeles home, ‘had injuries consistent with being stabbed’
-
 Hungary’s Krasznahorkai wins Nobel for literature
Hungary’s Krasznahorkai wins Nobel for literatureSpeed Read László Krasznahorkai is the author of acclaimed novels like ‘The Melancholy of Resistance’ and ‘Satantango’
-
 Primatologist Jane Goodall dies at 91
Primatologist Jane Goodall dies at 91Speed Read She rose to fame following her groundbreaking field research with chimpanzees
-
 Florida erases rainbow crosswalk at Pulse nightclub
Florida erases rainbow crosswalk at Pulse nightclubSpeed Read The colorful crosswalk was outside the former LGBTQ nightclub where 49 people were killed in a 2016 shooting
-
 Trump says Smithsonian too focused on slavery's ills
Trump says Smithsonian too focused on slavery's illsSpeed Read The president would prefer the museum to highlight 'success,' 'brightness' and 'the future'
-
 Trump to host Kennedy Honors for Kiss, Stallone
Trump to host Kennedy Honors for Kiss, StalloneSpeed Read Actor Sylvester Stallone and the glam-rock band Kiss were among those named as this year's inductees
