FDA approves test designed to measure specific COVID-19 antibody levels


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The Food and Drug Administration on Wednesday approved a "new generation" COVID-19 antibody test which is designed to tell how well people are protected against subsequent infection.
Most antibody tests are able to determine whether a person has contracted the virus or not, and some can estimate the level of neutralizing antibodies someone has, CNBC notes. But the newly-authorized COVID-SeroKlir developed by Kantaro BioSciences measures specific levels.
The catch is that scientists aren't sure if high levels of antibody guarantee COVID-19 immunity, so the test won't necessarily serve as proof that someone is protected against re-infection just yet. What it will do, however, is allow researchers to gain a better understanding about the correlation between antibody levels and immunity, which will come in handy both for people who have been exposed to the virus already and for vaccine development.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"It's going to broadly enable studies of immunity and the relationship between immunity and the level of antibodies that an individual has," said Erik Lium, the commercial innovation officer for the Mount Sinai Health System, which joined up with diagnostic startup Renalytix to form Kantaro. Read more at CNBC.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Tim is a staff writer at The Week and has contributed to Bedford and Bowery and The New York Transatlantic. He is a graduate of Occidental College and NYU's journalism school. Tim enjoys writing about baseball, Europe, and extinct megafauna. He lives in New York City.
-
 The EU’s war on fast fashion
The EU’s war on fast fashionIn the Spotlight Bloc launches investigation into Shein over sale of weapons and ‘childlike’ sex dolls, alongside efforts to tax e-commerce giants and combat textile waste
-
 How to Get to Heaven from Belfast: a ‘highly entertaining ride’
How to Get to Heaven from Belfast: a ‘highly entertaining ride’The Week Recommends Mystery-comedy from the creator of Derry Girls should be ‘your new binge-watch’
-
 The 8 best TV shows of the 1960s
The 8 best TV shows of the 1960sThe standout shows of this decade take viewers from outer space to the Wild West
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
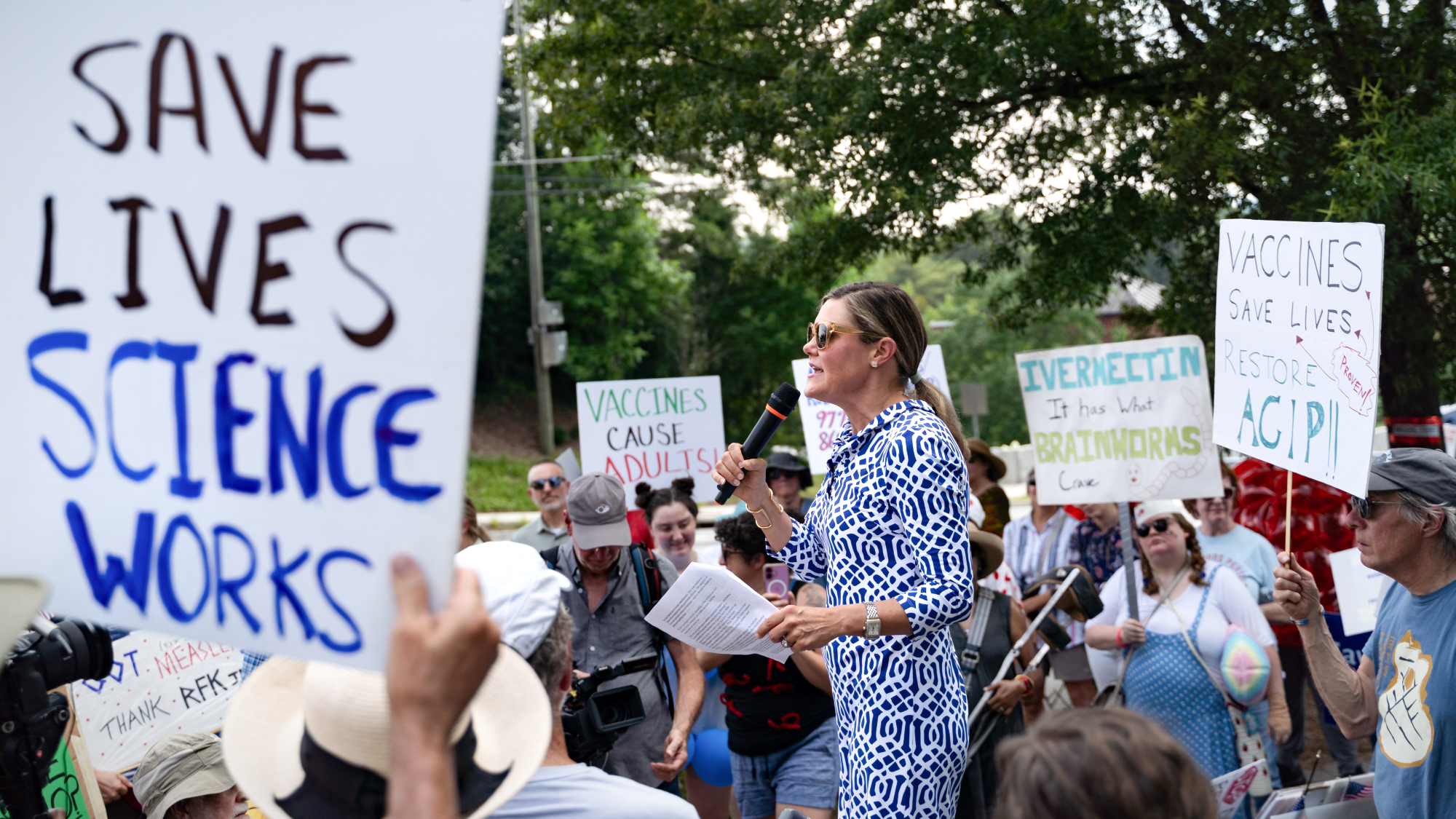 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
