Antidepressant used to treat OCD shows promise as COVID-19 early treatment

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The U.S. is now vaccinating more than 2 million adults a day against COVID-19, but "we know that vaccines are not going to reach everybody across the entire planet in the next couple of weeks," National Institutes of Health director Dr. Francis Collins told Sharyn Alfonsi on Sunday's 60 Minutes. "People are going to continue to get sick in the meantime," and "we need treatments for those people."
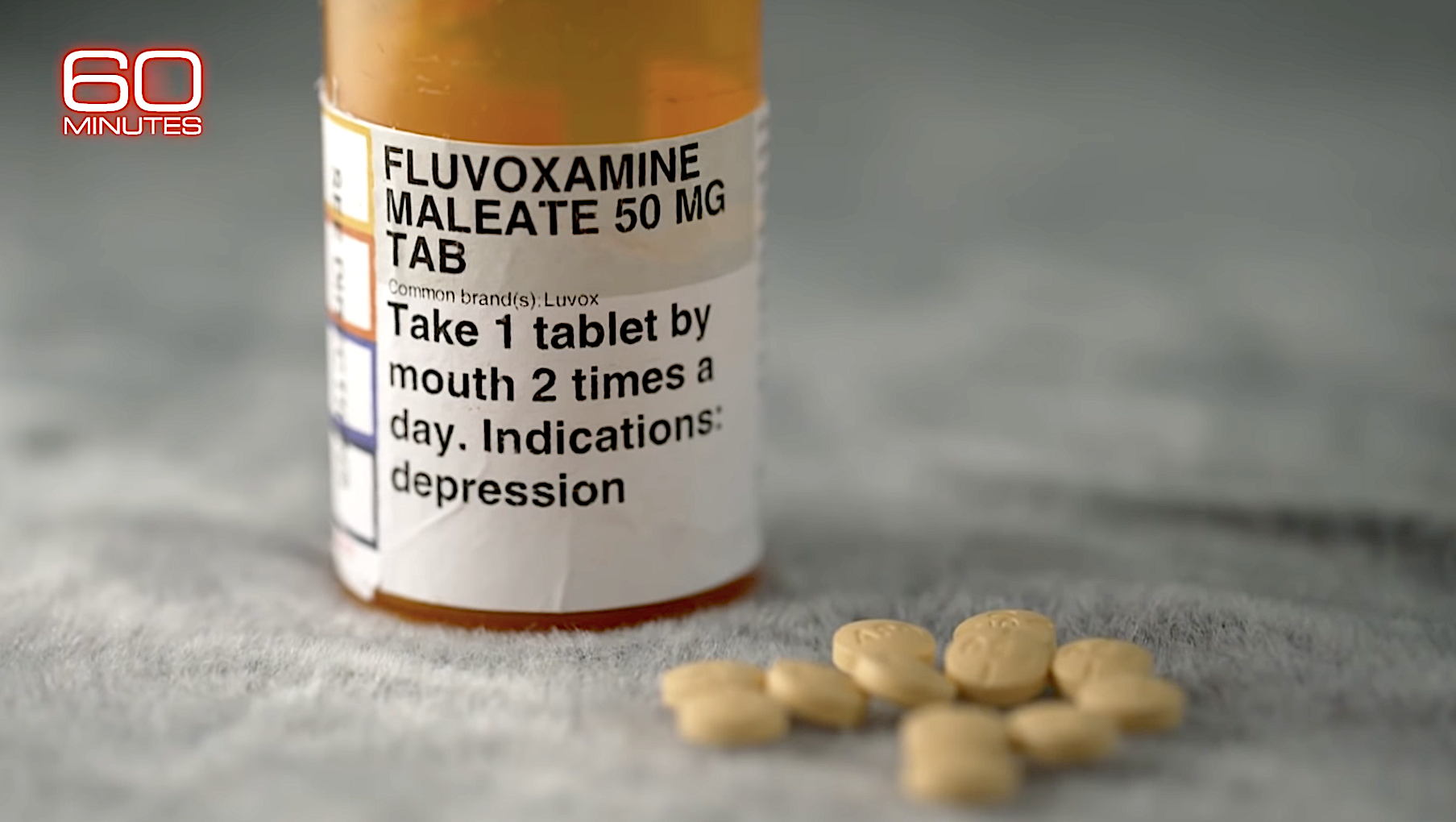
Specifically, Collins said, "a big need right now is for a drug that you can take by mouth that you could be offered as soon as you had a positive test and that would reduce the likelihood that that virus is going to make you very sick. And we have some very good clues there," one of them being the generic antidepressant fluvoxamine, developed 40 years ago and used most commonly to treat obsessive-compulsive disorder (OCD).

To explain how "a pill that costs 60 cents" became "a dark horse to treat COVID," 60 Minutes visited a race track in California, where Dr. David Seftel, working off a tip he received hours earlier, decided to offer his jockeys and other staff fluvoxamine to stem a COVID-19 growing outbreak at the track. "Sixty-five patients elected to take fluvoxamine; 49 declined," he told Alfonsi, and "12.5 percent of all those who refused fluvoxamine ended up hospitalized and one died. In the group that did take fluvoxamine, none of them were hospitalized."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.

Seftel had heard about fluvoxamine from Silicon Valley entrepreneur Steve Kirsch, who was funding a trial by Dr. Eric Lenze, a psychiatrist at Washington University in St. Louis, who in turn was tipped off by his colleague Dr. Angela Reiersen. In a small, methodologically sound trial published in the Journal of the American Medical Association (JAMA) in November, Lenze reported that none of the 80 of his 152 patients who took fluvoxamine after testing positive for COVID-19 deteriorated, versus 8 percent of the placebo-taking control group.

"So the results were really pretty incredible," Lenze told 60 Minutes. But "I have to be a scientist about this. We've tested it in one study. But — in my view, it needs to be confirmed in a larger study." That larger national study will report results starting next month. Collins told Alfonsi he regretted that last spring's "hydroxychloroquine debacle" sort of derailed the search for repurposed therapeutics, "but let me say, repurposing drugs is only going to work if you're kind of lucky." Peter Weber

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
