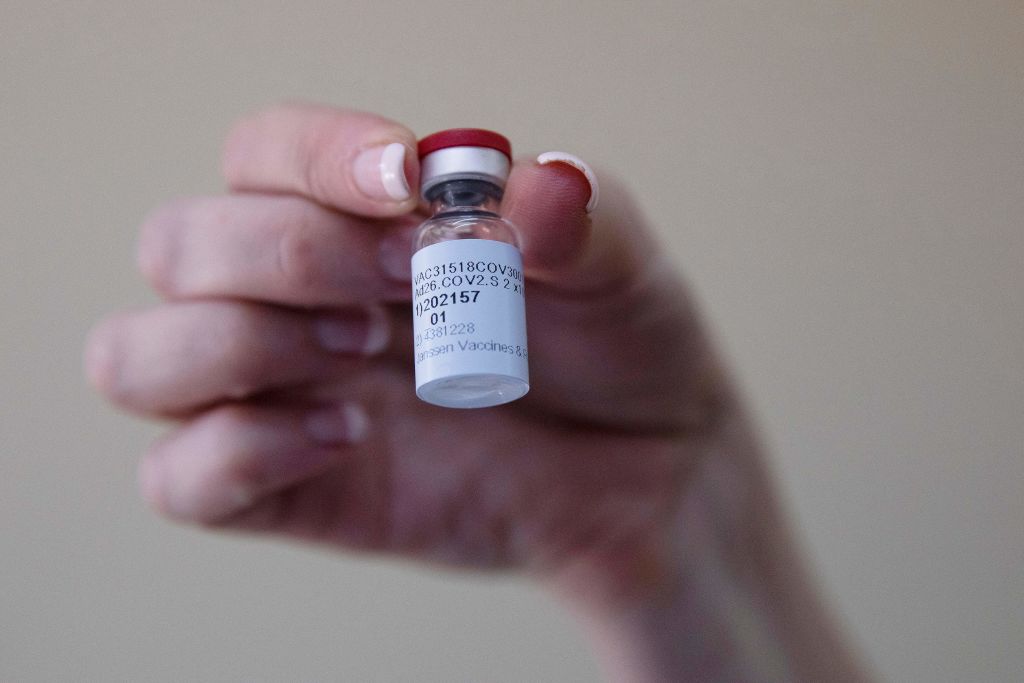
U.S. calls for Johnson & Johnson vaccine pause after 6 blood clotting cases

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The Food and Drug Administration and the Centers for Disease Control and Prevention are calling for a pause in the use of Johnson & Johnson's COVID-19 vaccine.
Officials from the two agencies on Tuesday said that "out of an abundance of caution," they're recommending the U.S. pause the use of the single-shot vaccine while they review "six reported U.S. cases of a rare and severe type of blood clot in individuals after receiving" it.
The six cases, officials said, were all in women who were between 18 and 48, with their symptoms having occurred between six and 13 days after they were vaccinated. Over 6.8 million doses of the Johnson & Johnson vaccine, which comes with the key benefit of only requiring one dose, have been administered in the United States, and the agencies noted that "right now, these adverse events appear to be extremely rare."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
On Wednesday, the CDC's Advisory Committee on Immunization Practices is set to hold an emergency meeting to review the clotting cases. The federal government will also stop administering the vaccine at federally run vaccination sites, The New York Times reported. This pause, the Times noted, "could substantially complicate" the United States' vaccination efforts.
The decision drew some immediate criticism given the small number of blood clotting cases, with FiveThirtyEight's Nate Silver raising concerns that it's "going to create more vaccine hesitancy." But the FDA's Dr. Peter Marks and the CDC's Dr. Anne Schuchat said the step is "important, in part, to ensure that the health care provider community is aware of the potential for these adverse events and can plan for proper recognition and management due to the unique treatment required with this type of blood clot."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brendan worked as a culture writer at The Week from 2018 to 2023, covering the entertainment industry, including film reviews, television recaps, awards season, the box office, major movie franchises and Hollywood gossip. He has written about film and television for outlets including Bloody Disgusting, Showbiz Cheat Sheet, Heavy and The Celebrity Cafe.
