CDC clears COVID booster shots for seniors, at-risk, and — siding with FDA over advisory panel — frontline workers


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
The Centers for Disease Control and Prevention late Thursday gave final authorization for tens of millions of Americans to get a COVID-19 booster shot — but it sided with the Food and Drug Administration over its own advisory panel in approving a third dose of the Pfizer-BioNTech vaccine for people 18 and older who are at greater risk of infection because of their jobs. The other groups that can now sign up for booster shots are people 65 and older, those living in long-term care facilities, and adults 50 and older who have underlying health conditions.
CDC Director Rochelle Walensky suggested Thursday night that she overruled the Advisory Committee on Immunization Practices regarding frontline workers because it's her job "to recognize where our actions can have the greatest impact," and based on the "complex, often imperfect data," the CDC "must take actions that we anticipate will do the greatest good." The authorization only applies to a third Pfizer shot at least six months after a person's second dose, but Walensky said the CDC "will address, with the same sense of urgency, recommendations for the Moderna and J & J vaccines as soon as those data are available."
The CDC immunization advisory panel had agreed with the FDA on most booster populations it authorized Wednesday night, but it voted 9 to 6 against recommending third shots for health-care providers, teachers, prison guards, grocery store workers, and others whose "frequent institutional or occupational exposure" puts them at greater risk.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Some panel members said they voted against boosters for frontline workers because it opened the door to too many people or gave the false impression that the vaccines aren't still incredibly effective at protecting most people against serious illness, hospitalization, and death. Others suggested boosters might take focus off the primary goal of getting unvaccinated people their first shots.
Biden administration officials had quietly hoped the CDC would side with the FDA, both so the nation's two top public health agencies would be in accord and also because President Biden and his advisers had wanted the booster shots cleared for most vaccinated Americans. In any case, "in reality, anyone who wants a booster will get one, as has already been happening," a federal health official told The Washington Post. More than two-thirds of COVID vaccinations are administered at pharmacies, and people don't need prescriptions or other documentation to get jabbed.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Peter has worked as a news and culture writer and editor at The Week since the site's launch in 2008. He covers politics, world affairs, religion and cultural currents. His journalism career began as a copy editor at a financial newswire and has included editorial positions at The New York Times Magazine, Facts on File, and Oregon State University.
-
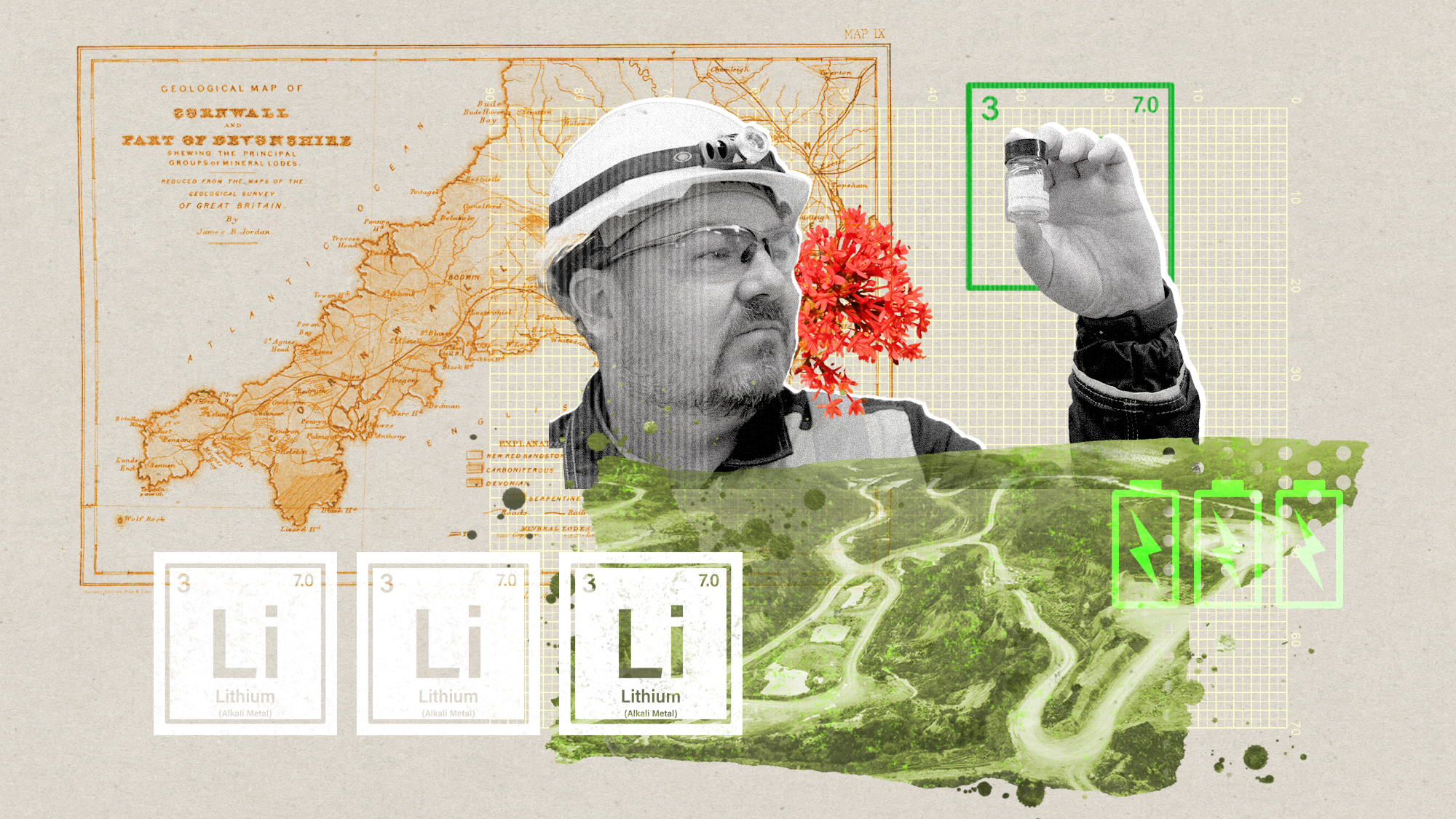 The ‘ravenous’ demand for Cornish minerals
The ‘ravenous’ demand for Cornish mineralsUnder the Radar Growing need for critical minerals to power tech has intensified ‘appetite’ for lithium, which could be a ‘huge boon’ for local economy
-
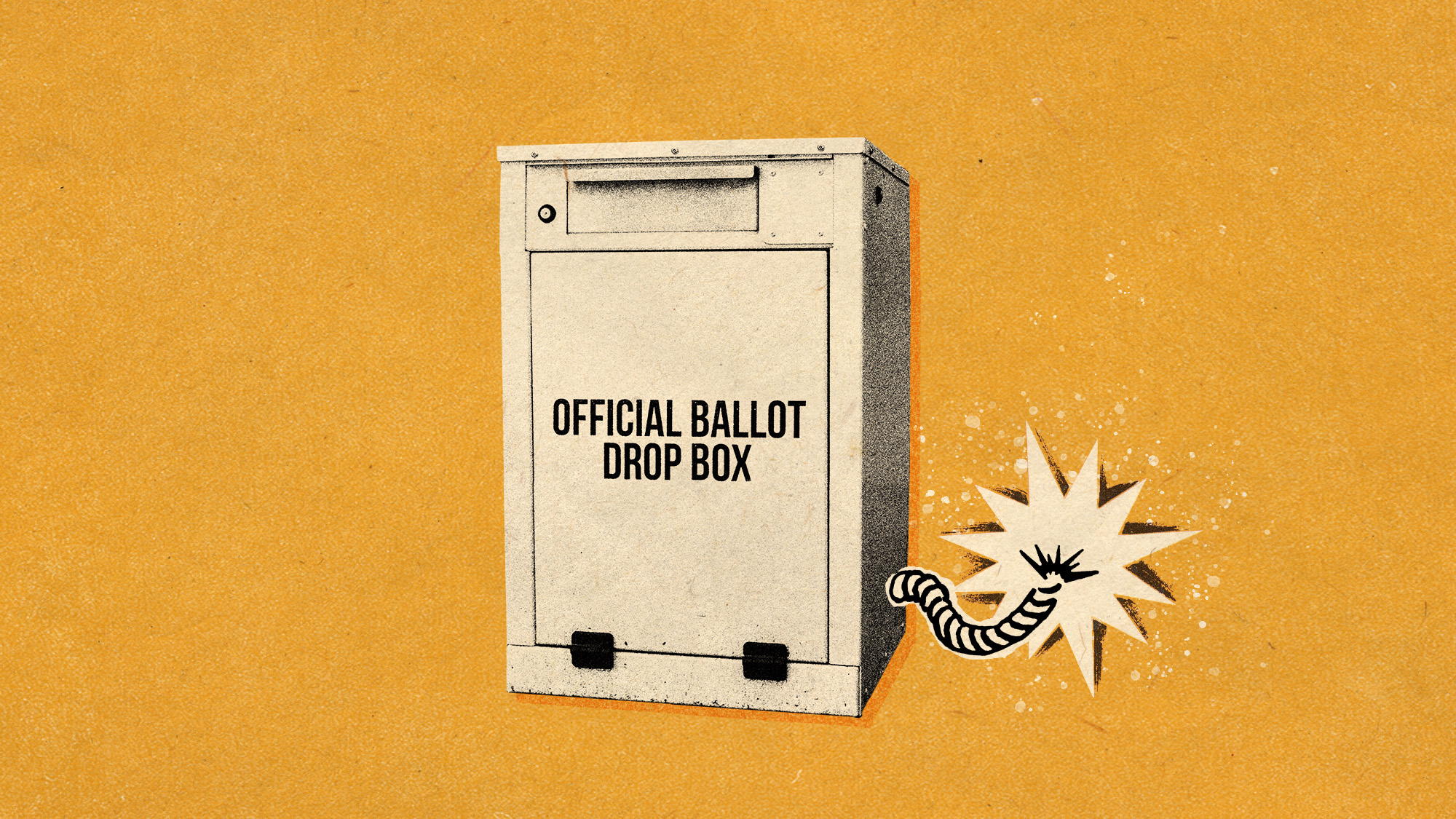 Why are election experts taking Trump’s midterm threats seriously?
Why are election experts taking Trump’s midterm threats seriously?IN THE SPOTLIGHT As the president muses about polling place deployments and a centralized electoral system aimed at one-party control, lawmakers are taking this administration at its word
-
 ‘Restaurateurs have become millionaires’
‘Restaurateurs have become millionaires’Instant Opinion Opinion, comment and editorials of the day
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
 How dangerous is the ‘K’ strain super-flu?
How dangerous is the ‘K’ strain super-flu?The Explainer Surge in cases of new variant H3N2 flu in UK and around the world
-
 Vaccine critic quietly named CDC’s No. 2 official
Vaccine critic quietly named CDC’s No. 2 officialSpeed Read Dr. Ralph Abraham joins another prominent vaccine critic, HHS Secretary Robert F. Kennedy Jr.
-
 This flu season could be worse than usual
This flu season could be worse than usualIn the spotlight A new subvariant is infecting several countries
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system
