Ebola vaccine: first large-scale trial begins in West Africa
Liberia has received the first batch, but experts warn it may be difficult to establish its efficacy
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
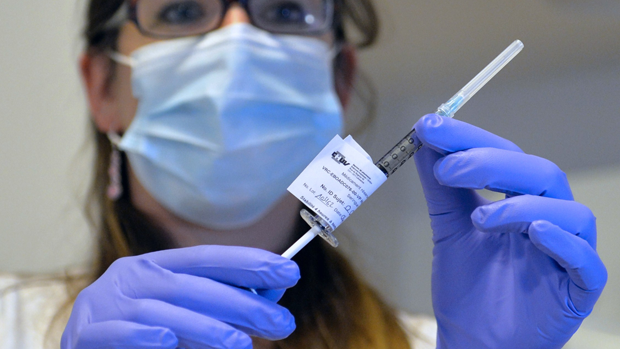
The first large-scale trial of an experimental Ebola vaccine is to begin today in one of the West African countries worst affected by the disease, following a successful preliminary safety trial.
The batch of vaccines, developed by the British pharmaceutical giant GlaxoSmithKline, was transported under tight security to an unknown location in Liberia, the BBC reports. Up to 30,000 volunteers in the country are expected to be involved in the trial, with a third receiving the vaccine.
The vaccine contains a small amount of the virus which scientists hope will trigger an immune response that will protect against infection.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Stephen Kennedy, the senior Liberian scientist involved in the trials said that the vaccine posed no risk to volunteers. "It is a weak strain and it cannot and will not cause Ebola, so it is impossible that any one of the volunteers will contact Ebola from the vaccine," he said.
GlaxoSmithKline's global vaccines chief Dr Moncef Slaoui described the shipment as "a major achievement", which "shows that we remain on track" with accelerated development of the Ebola vaccine.
"The initial phase one data... are encouraging and give us confidence to progress to the next phases of clinical testing, which will involve the vaccination of thousands of volunteers, including frontline healthcare workers," he said.
The company stressed, however, that the vaccine was still in its development phase and its long-term safety and efficacy would have to be established before it could be used on a wider scale.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
"Any potential future use in mass vaccination campaigns will depend on whether the World Health Organization (WHO) regulators and other stakeholders are satisfied... and how quickly large quantities... can be made," said Slaoui.
Last month, the WHO said that the outbreak in West Africa had reached a turning point, as the number of new infections continued to decline, but warned against complacency in the fight to eradicate the disease entirely.
Paradoxically, this encouraging news may have a negative impact on the vaccine trial. "Because case numbers are starting to come down, it will become harder and harder to show if the vaccine is having any impact," Professor Jonathan Ball, a virus expert based at Nottingham University, told the BBC.
There is currently no proven vaccine or cure for Ebola and the unprecedented scale of the outbreak prompted governments, pharmaceutical companies and international health organisations to fast track the development of safe and effective treatments.
There are several other promising vaccines and treatments currently in development, with reports that the experimental drug zMapp, which was given to several Western healthcare workers who later recovered, will be trialled in the coming weeks.
The deadliest Ebola outbreak in history has so far claimed at least 8,641 lives and almost 22,000 people have been confirmed infected, although that number is expected to be much higher in reality.
-
 Tourangelle-style pork with prunes recipe
Tourangelle-style pork with prunes recipeThe Week Recommends This traditional, rustic dish is a French classic
-
 The Epstein files: glimpses of a deeply disturbing world
The Epstein files: glimpses of a deeply disturbing worldIn the Spotlight Trove of released documents paint a picture of depravity and privilege in which men hold the cards, and women are powerless or peripheral
-
 Jeff Bezos: cutting the legs off The Washington Post
Jeff Bezos: cutting the legs off The Washington PostIn the Spotlight A stalwart of American journalism is a shadow of itself after swingeing cuts by its billionaire owner
-
 Epstein files topple law CEO, roil UK government
Epstein files topple law CEO, roil UK governmentSpeed Read Peter Mandelson, Britain’s former ambassador to the US, is caught up in the scandal
-
 Iran and US prepare to meet after skirmishes
Iran and US prepare to meet after skirmishesSpeed Read The incident comes amid heightened tensions in the Middle East
-
 Israel retrieves final hostage’s body from Gaza
Israel retrieves final hostage’s body from GazaSpeed Read The 24-year-old police officer was killed during the initial Hamas attack
-
 China’s Xi targets top general in growing purge
China’s Xi targets top general in growing purgeSpeed Read Zhang Youxia is being investigated over ‘grave violations’ of the law
-
 Panama and Canada are negotiating over a crucial copper mine
Panama and Canada are negotiating over a crucial copper mineIn the Spotlight Panama is set to make a final decision on the mine this summer
-
 Why Greenland’s natural resources are nearly impossible to mine
Why Greenland’s natural resources are nearly impossible to mineThe Explainer The country’s natural landscape makes the task extremely difficult
-
 Iran cuts internet as protests escalate
Iran cuts internet as protests escalateSpeed Reada Government buildings across the country have been set on fire
-
 US nabs ‘shadow’ tanker claimed by Russia
US nabs ‘shadow’ tanker claimed by RussiaSpeed Read The ship was one of two vessels seized by the US military