Ebola: experimental vaccine trial an 'unqualified success'
Early human tests suggest the drug is safe and may help immune systems fight Ebola, but more trials are needed
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
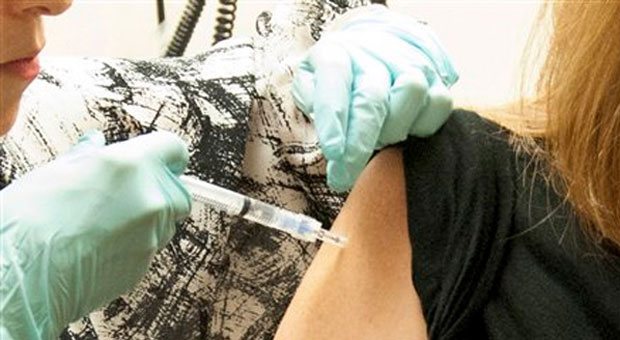
The first human trial of an experimental Ebola vaccine has delivered promising results, according to the US National Institute of Health (NIH).
"On safety and on the ability to produce an appropriate immune response we can call this trial an unqualified success," Dr Anthony Fauci of the NIH told the BBC.
However, he stressed that this was only the early part of the first phase of the trial and much more testing was required to establish the vaccine's long-term safety and efficacy.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
There is currently no vaccine or cure for Ebola and the unprecedented scale of the current outbreak in West Africa has prompted pharmaceutical companies and health organisations to fast track the development of safe and effective treatments.
The vaccine has been jointly produced by the NIH and the British pharmaceutical company GlaxoSmithKline (GSK) and is one of three currently in development.
In the first phase of the trial, all 20 American volunteers who received the vaccine developed antibodies to the virus and none suffered any major side effects. However, some patients did develop a fever in response to the drug, but it "resolved within one day", according to scientists.
GSK has reportedly made a request for an indemnity agreement to protect itself against unforeseen side effects that could develop in the future.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
"It's important to remember that these data are the first piece in the jigsaw and we're continuing to gather other important information," Dr Moncef Slaoui, chairman of global vaccines at GSK told The Guardian.
If the results of the following phases of the trial are positive, thousands of volunteers, including healthcare workers, will receive the vaccine in West Africa in early 2015, he said.
Almost 5,700 people are known to have died from the disease and almost 15,000 have been infected across the region, though actual figures are believed to be much higher.