FDA issues 'transformative' authorization of Pfizer's COVID vaccine for children ages 5 to 11

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
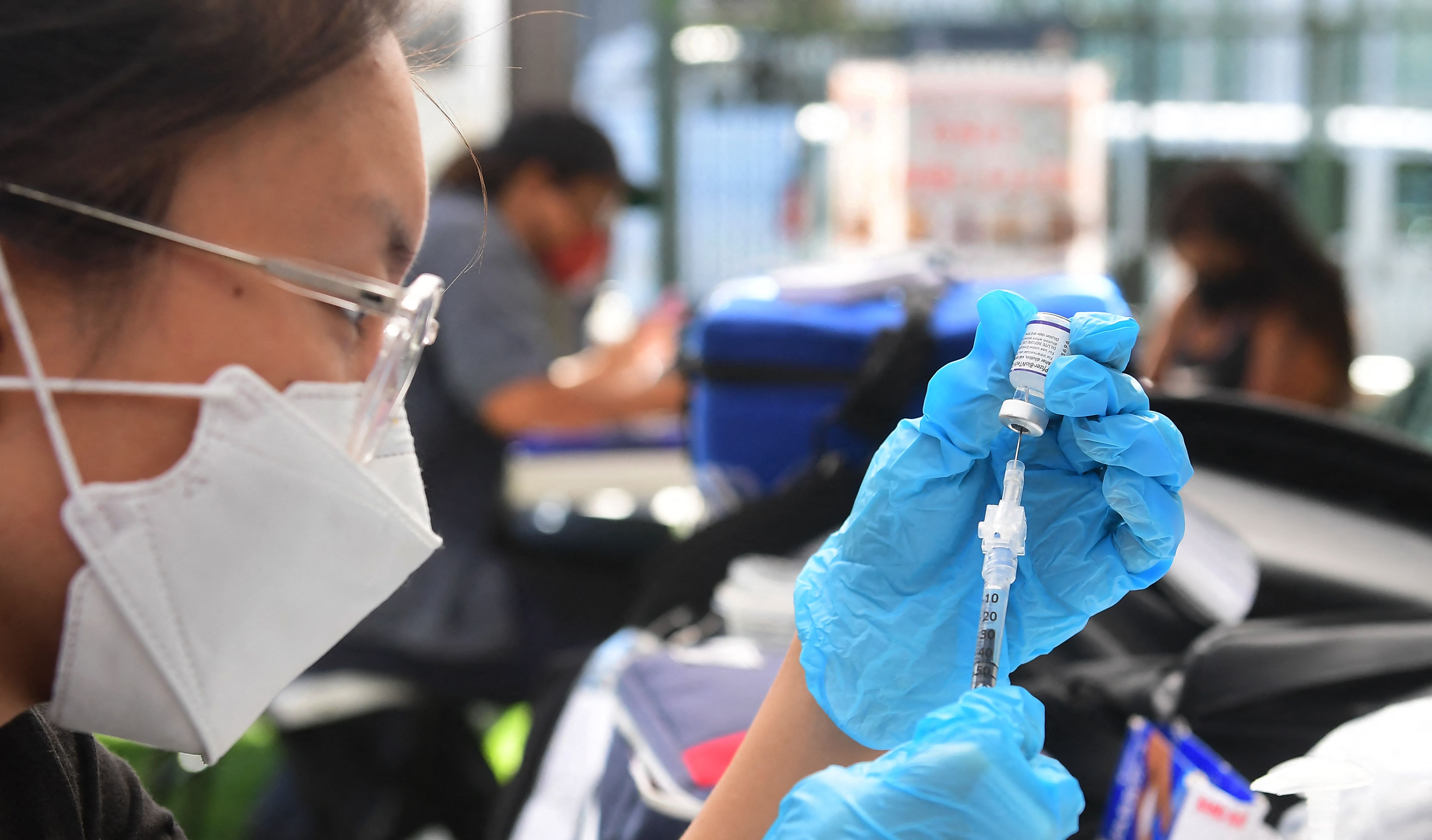
The Food and Drug Administration moved to give Pfizer's COVID-19 vaccine emergency use authorization for American children ages 5 to 11. The Friday decision, which was expected, was heralded as a "watershed moment," "transformative news" for parents, and a "hotly anticipated" milestone in the effort to end the pandemic.
The Pfizer-BioNTech vaccine got the green light from the FDA, and now just needs to be recommended by the Centers for Disease Control and Prevention. That recommendation may come shortly after a Nov. 2 CDC committee meeting.
Roughly 28 million American children will be allowed to be vaccinated once the authorization is finalized, though The Washington Post notes that officials are expecting some level of hesitation from parents who may prefer to wait for full FDA approval or agree with some advisory panelists who argued not all children need the vaccine, because the risk of COVID-19 complications is lower in most kids. However, polling has showed most parents will welcome the vaccine for their children. Stat News notes the Pfizer vaccine was found to be almost 91 percent effective in preventing COVID-19 in kids ages 5 to 11, and didn't cause any serious complications.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy," said acting FDA Commissioner Janet Woodcock. "Our comprehensive and rigorous evaluation of the data pertaining to the vaccine's safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards."
If and when CDC Director Rochelle Walensky signs off on emergency authorization for the age group next week, providers will be able to begin administering shots same-day.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Summer Meza has worked at The Week since 2018, serving as a staff writer, a news writer and currently the deputy editor. As a proud news generalist, she edits everything from political punditry and science news to personal finance advice and film reviews. Summer has previously written for Newsweek and the Seattle Post-Intelligencer, covering national politics, transportation and the cannabis industry.
-
 5 calamitous cartoons about the Washington Post layoffs
5 calamitous cartoons about the Washington Post layoffsCartoons Artists take on a new chapter in journalism, democracy in darkness, and more
-
 Political cartoons for February 14
Political cartoons for February 14Cartoons Saturday's political cartoons include a Valentine's grift, Hillary on the hook, and more
-
 Tourangelle-style pork with prunes recipe
Tourangelle-style pork with prunes recipeThe Week Recommends This traditional, rustic dish is a French classic
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
 How dangerous is the ‘K’ strain super-flu?
How dangerous is the ‘K’ strain super-flu?The Explainer Surge in cases of new variant H3N2 flu in UK and around the world
-
 Vaccine critic quietly named CDC’s No. 2 official
Vaccine critic quietly named CDC’s No. 2 officialSpeed Read Dr. Ralph Abraham joins another prominent vaccine critic, HHS Secretary Robert F. Kennedy Jr.
-
 This flu season could be worse than usual
This flu season could be worse than usualIn the spotlight A new subvariant is infecting several countries
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system
