FDA clears Moderna, Pfizer vaccines for kids as young as 6 months

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
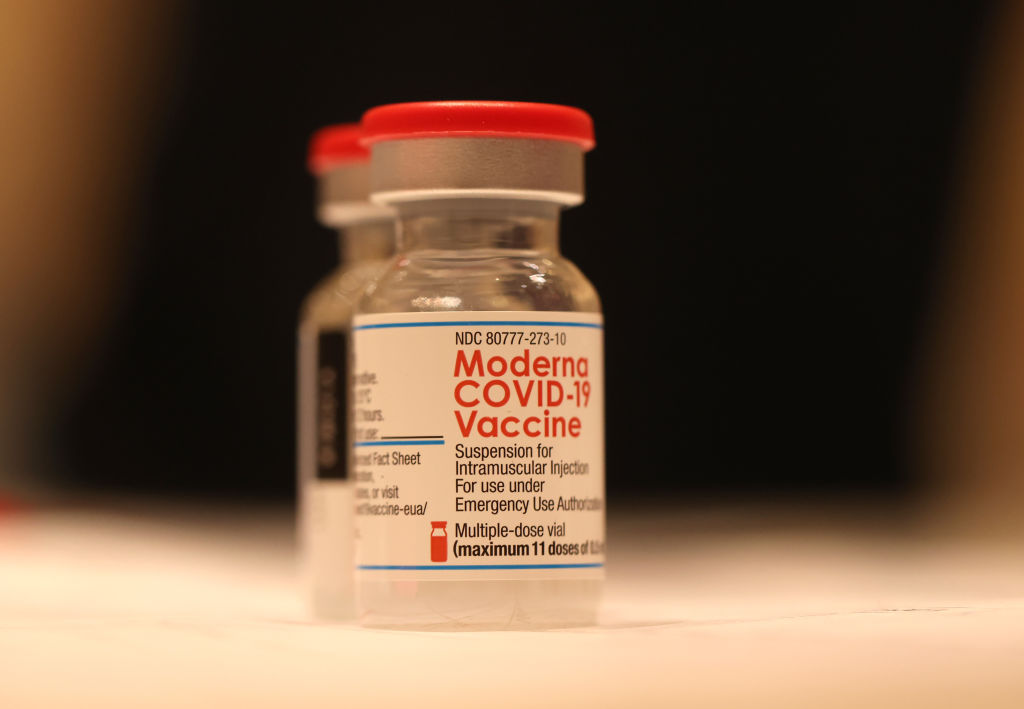
The U.S. Food and Drug Administration on Friday authorized both Moderna's and Pfizer's COVID-19 vaccines for emergency use in kids as young as 6 months, bringing the U.S. one giant step closer to protecting its youngest citizens against the coronavirus.
The agency's decision arrived after a group of independent advisers voted unanimously on Wednesday to recommend the shots. Pfizer's three-dose regimen has now been authorized for use in children ages 6 months to 4 years old, while Moderna's two-dose regimen is now cleared for children ages 6 months to 5 years old.
Before vaccinations can officially begin, however, Centers for Disease Control and Prevention Director Rochelle Walensky must issue her own recommendation, which will likely happen following a vaccine advisory panel vote this weekend. The White House is expecting shots to begin as soon as Tuesday, CNBC reports.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
"As we have seen with older age groups," said FDA Commissioner Dr. Robert Califf, "we expect that the vaccines for younger children will provide protection from the most severe outcomes of COVID-19, such as hospitalization and death."
The FDA also on Friday authorized Moderna's vaccine for kids ages 6 through 17; previously, only the shot from Pfizer-BioNTech was cleared for that age group.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brigid Kennedy worked at The Week from 2021 to 2023 as a staff writer, junior editor and then story editor, with an interest in U.S. politics, the economy and the music industry.
-
 ‘Poor time management isn’t just an inconvenience’
‘Poor time management isn’t just an inconvenience’Instant Opinion Opinion, comment and editorials of the day
-
 Bad Bunny’s Super Bowl: A win for unity
Bad Bunny’s Super Bowl: A win for unityFeature The global superstar's halftime show was a celebration for everyone to enjoy
-
 Book reviews: ‘Bonfire of the Murdochs’ and ‘The Typewriter and the Guillotine’
Book reviews: ‘Bonfire of the Murdochs’ and ‘The Typewriter and the Guillotine’Feature New insights into the Murdoch family’s turmoil and a renowned journalist’s time in pre-World War II Paris
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 The new Stratus Covid strain – and why it’s on the rise
The new Stratus Covid strain – and why it’s on the riseThe Explainer ‘No evidence’ new variant is more dangerous or that vaccines won’t work against it, say UK health experts
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
