FDA authorizes Omicron-specific boosters

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
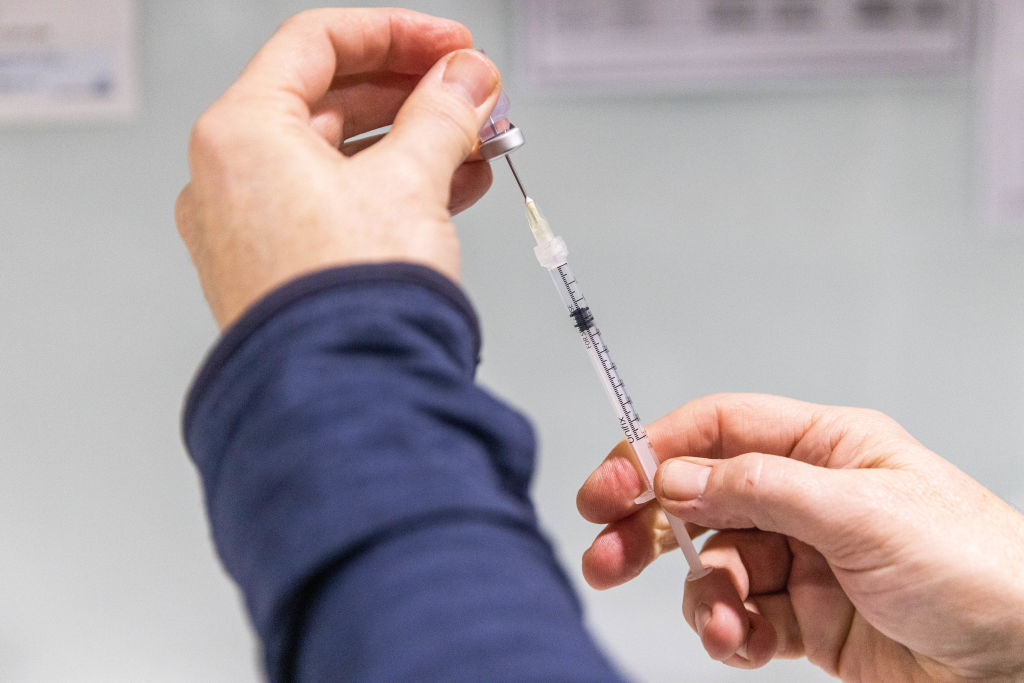
The U.S. Food and Drug Administration on Wednesday authorized updated, Omicron-specific COVID-19 boosters from both Pfizer and Moderna, multiple outlets report.
The new shots are expected post-Labor Day, pending review from the Centers for Disease Control and Prevention later this week, notes The Washington Post. The boosters' bivalent formula targets both the BA.4 and BA.5 Omicron subvariant, as well as the original strain of the virus that circulated back in 2020.
The agency notably cleared the jabs without data from human trials, which it argued could detrimentally slow the process and perhaps render moot any data used in authorization, per The New York Times.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Pfizer's booster was cleared for use in individuals ages 12 and up, while the Moderna shot was authorized for those 18 and older. Prospective recipients must wait at least two months after finishing their primary series of vaccinations or after receiving their last booster dose to get the new shot.
"As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose," FDA Commissioner Robert Califf said Wednesday.
The Biden administration is preparing to distribute the shots as part of its fall booster campaign, NBC News adds.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brigid Kennedy worked at The Week from 2021 to 2023 as a staff writer, junior editor and then story editor, with an interest in U.S. politics, the economy and the music industry.
-
 Crisis in Cuba: a ‘golden opportunity’ for Washington?
Crisis in Cuba: a ‘golden opportunity’ for Washington?Talking Point The Trump administration is applying the pressure, and with Latin America swinging to the right, Havana is becoming more ‘politically isolated’
-
 5 thoroughly redacted cartoons about Pam Bondi protecting predators
5 thoroughly redacted cartoons about Pam Bondi protecting predatorsCartoons Artists take on the real victim, types of protection, and more
-
 Palestine Action and the trouble with defining terrorism
Palestine Action and the trouble with defining terrorismIn the Spotlight The issues with proscribing the group ‘became apparent as soon as the police began putting it into practice’
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 The new Stratus Covid strain – and why it’s on the rise
The new Stratus Covid strain – and why it’s on the riseThe Explainer ‘No evidence’ new variant is more dangerous or that vaccines won’t work against it, say UK health experts
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
