Solving COVID: January 27, 2021
Biden orders 200 million more vaccine doses, Merck abandons vaccine plans, and more

- 1. Biden administration orders another 200 million vaccine doses
- 2. Inadequate immune responses force Merck to abandon COVID-19 vaccines
- 3. Moderna working on booster shot to increase protection against South African coronavirus variant
- 4. Cancer drug is 27.5 times more effective at treating COVID-19 than remdesivir, study suggests
- 5. Eli Lilly says trial showed its COVID-19 drug reduced hospitalizations
1. Biden administration orders another 200 million vaccine doses
The Biden administration has ordered another 200 million coronavirus vaccine doses from Pfizer and Moderna, President Biden said Tuesday. Adding the new purchase to the 400 million doses already ordered, the U.S. should have enough vaccine to inoculate every American who wants a vaccine by the end of summer. "It will be enough to fully vaccinate 300 million Americans to beat the pandemic," Biden said. The Centers for Disease Control and Prevention said that 23.5 million doses had been administered in the U.S. as of Tuesday, with more than 3.4 million people fully vaccinated. Vaccine doses shipped to states are due to increase by 20 percent to 10 million per week, a Biden administration official said. Johnson & Johnson plans to release data on its single-dose vaccine, which soon could add to the supply.
2. Inadequate immune responses force Merck to abandon COVID-19 vaccines
Merck is abandoning the development of its two COVID-19 vaccines after initial trials resulted in inadequate immune responses. Both vaccines produced lower levels of coronavirus antibodies than have been found in the blood of individuals who recovered from natural COVID-19 infections. In comparison, the Pfizer-BioNTech and Moderna vaccines produced antibody levels several times higher than natural infections. The unsuccessful trials are considered disappointing in large part because both Merck vaccines would have required just one dose. One of the two candidates, which uses the same virus as the one in Merck's successful Ebola vaccine, was being developed in partnership with the International AIDS Vaccine Initiative, which has said it will try to determine if using an oral or intranasal administration route will work better than the current intramuscular injection, though Merck has suggested it will turn its attention to COVID-19 therapeutics.
Subscribe to The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
3. Moderna working on booster shot to increase protection against South African coronavirus variant
Moderna is working on a booster shot to ensure its vaccine is effective against the COVID-19 variant first found in South Africa. More transmissible variants of COVID-19 first appeared overseas last month, and have since been found in the U.S. While Moderna's vaccine was found effective against the more transmissible B117 variant first discovered in London, a study released Monday showed the vaccine saw a sixfold reduction in antibodies produced when used against the B1351 strain from South Africa. Moderna affirmed the antibodies produced with the vaccine "remain above levels that are expected to be protective" against either strain. But "as an insurance policy," the company is developing a booster shot for the South Africa-based strain, Moderna's chief medical officer Dr. Tal Zaks told The New York Times.
The New York Times The Wall Street Journal
4. Cancer drug is 27.5 times more effective at treating COVID-19 than remdesivir, study suggests
Researchers reported in the journal Science that a drug developed to fight multiple myeloma has proved 27.5 times more effective at treating COVID-19 than remdesivir in laboratory studies with infected human lung and kidney cells. The drug, Aplidin or Plitidepsin, was also effective at fighting COVID-19 in lab mice. Aplidin was developed in Spain from a tubular, plantlike marine animal called a sea squirt. It has gone through a Phase II trial against COVID-19 and is awaiting a Phase III trial. Researchers at the University of California, San Francisco, started exploring Aplidin's use as a treatment for COVID-19 in March. Instead of looking through databases of existing drugs to find one that targeted the virus, the UC San Francisco team sought out drugs that would protect key human proteins from being hijacked by the coronavirus. Experts not involved in the study said the research was promising but needed confirmation in human trials.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
5. Eli Lilly says trial showed its COVID-19 drug reduced hospitalizations
Eli Lilly announced Tuesday that its coronavirus drug bamlanivimab reduced hospitalizations for infected, high-risk patients by 70 percent in a large, late-stage trial. The drug was approved for emergency use last year by the Food and Drug Administration. It has been given to 125,000 high risk patients across the United States, including former President Donald Trump, former New Jersey Gov. Chris Christie, and Rudy Giuliani, a former New York City mayor and Trump's personal attorney. Eli Lilly said that 10 percent of the COVID-19 patients given a placebo in its trial wound up being hospitalized, compared to just 2 percent of those given bamlanivimab combined with another monoclonal antibody, etesevimab. None of the patients given the cocktail died, but eight given the placebo did. Patients given bamlanivimab alone also did well.
-
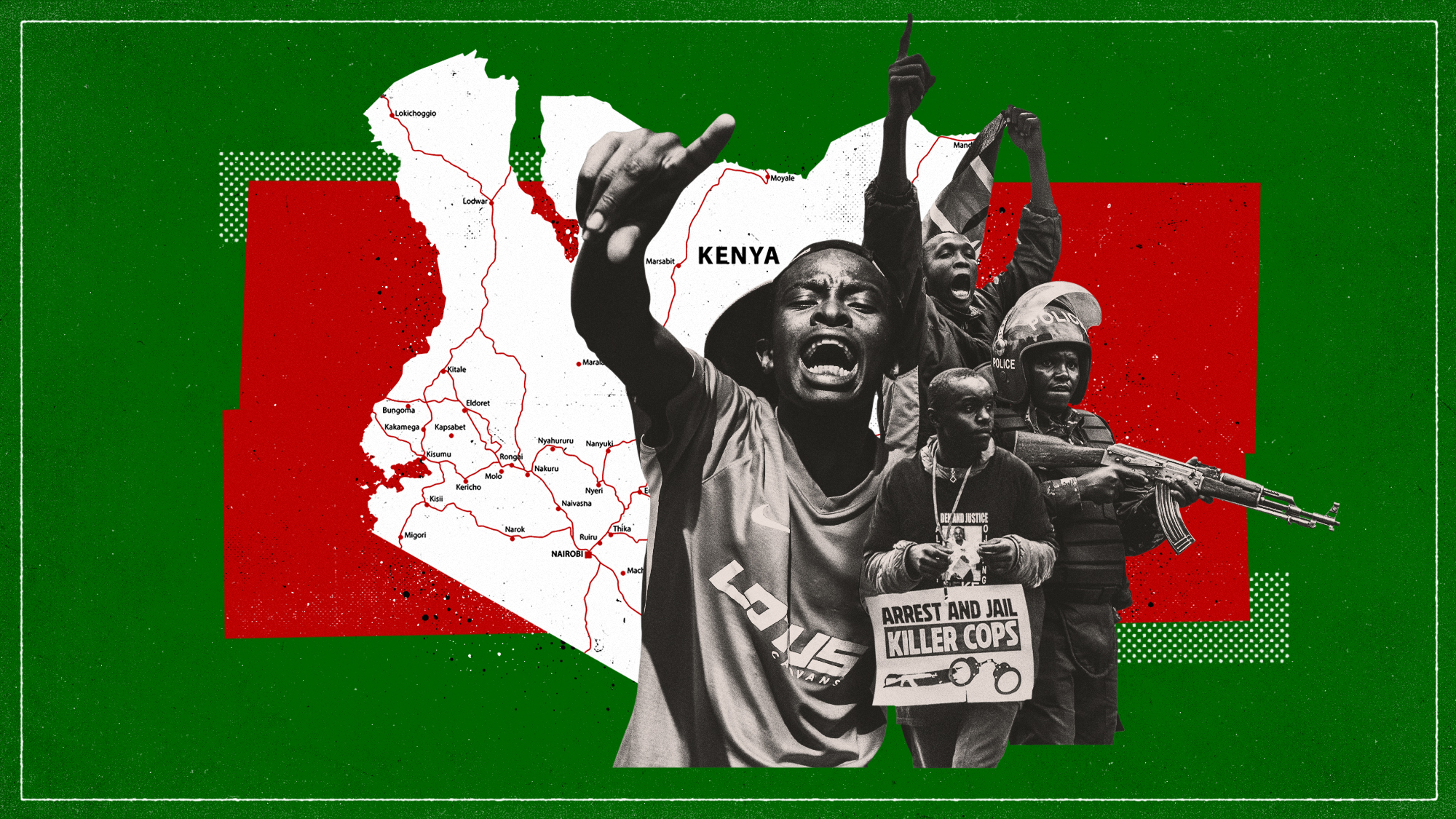 One year after mass protests, why are Kenyans taking to the streets again?
One year after mass protests, why are Kenyans taking to the streets again?today's big question More than 60 protesters died during demonstrations in 2024
-
 What happens if tensions between India and Pakistan boil over?
What happens if tensions between India and Pakistan boil over?TODAY'S BIG QUESTION As the two nuclear-armed neighbors rattle their sabers in the wake of a terrorist attack on the contested Kashmir region, experts worry that the worst might be yet to come
-
 Why Russia removed the Taliban's terrorist designation
Why Russia removed the Taliban's terrorist designationThe Explainer Russia had designated the Taliban as a terrorist group over 20 years ago
-
 Inside the Israel-Turkey geopolitical dance across Syria
Inside the Israel-Turkey geopolitical dance across SyriaTHE EXPLAINER As Syria struggles in the wake of the Assad regime's collapse, its neighbors are carefully coordinating to avoid potential military confrontations
-
 'Like a sound from hell': Serbia and sonic weapons
'Like a sound from hell': Serbia and sonic weaponsThe Explainer Half a million people sign petition alleging Serbian police used an illegal 'sound cannon' to disrupt anti-government protests
-
 The arrest of the Philippines' former president leaves the country's drug war in disarray
The arrest of the Philippines' former president leaves the country's drug war in disarrayIn the Spotlight Rodrigo Duterte was arrested by the ICC earlier this month
-
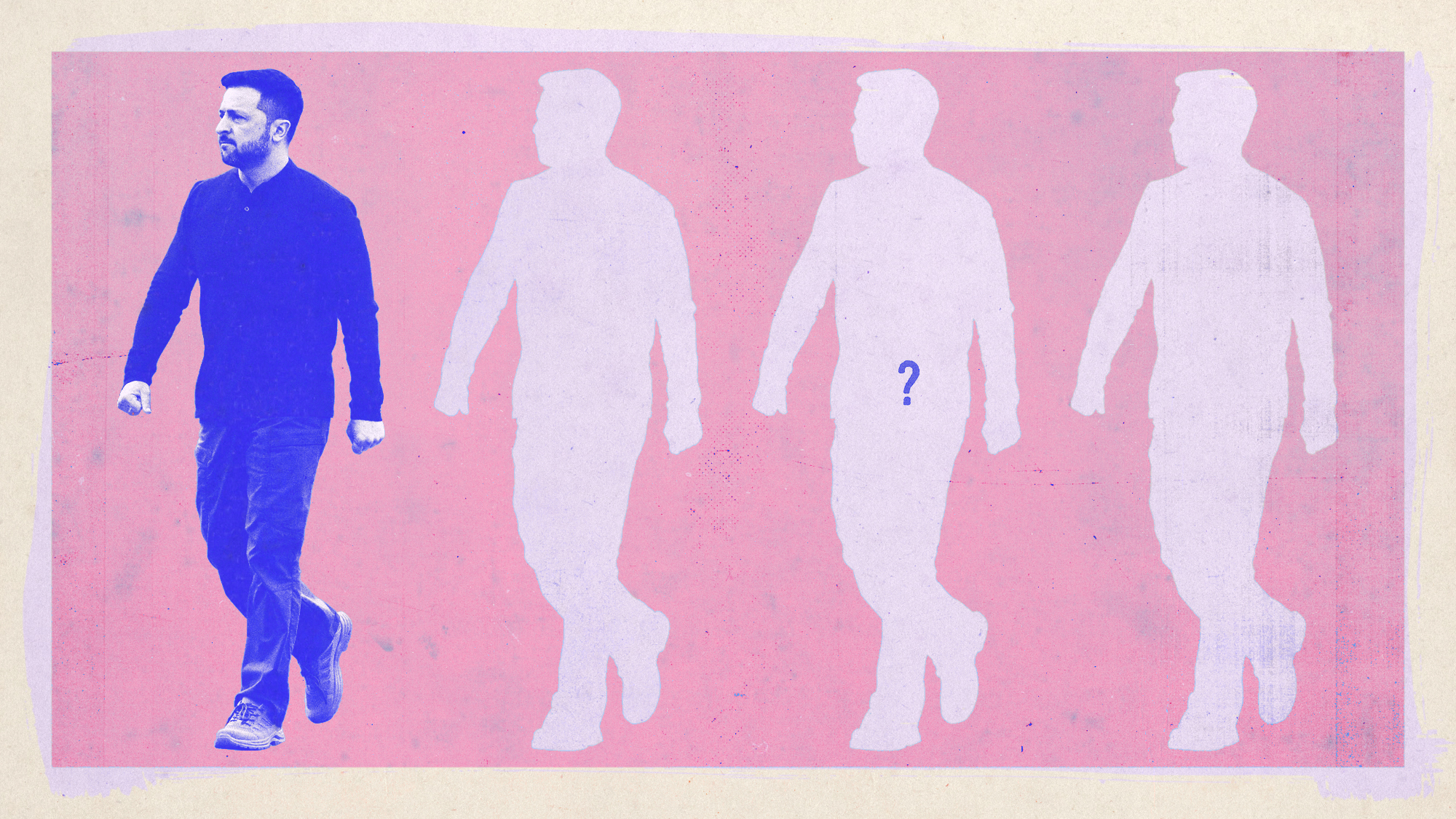 Ukrainian election: who could replace Zelenskyy?
Ukrainian election: who could replace Zelenskyy?The Explainer Donald Trump's 'dictator' jibe raises pressure on Ukraine to the polls while the country is under martial law
-
 Why Serbian protesters set off smoke bombs in parliament
Why Serbian protesters set off smoke bombs in parliamentTHE EXPLAINER Ongoing anti-corruption protests erupted into full view this week as Serbian protesters threw the country's legislature into chaos


