Lecanemab: Understanding the controversial new Alzheimer's drug
Is lecanemab worth the risk?

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
There is some promising news in the fight against Alzheimer's disease. The makers of a new drug, lecanemab, announced that trial studies demonstrate it reduces the rate of cognitive decline in Alzheimer's patients by 27 percent. The news "comes after many years of frustration and failure" by researchers looking to slow and reverse the disease, NPR reports.
But there is a downside: The trials also show lecanemab has "significant safety risks," The New York Times reports. Two patients who took the drug died after experiencing brain swelling and brain bleeding. "The benefit is real; so too are the risks," a University of Pennsylvania doctor told the newspaper. So is the drug worth those risks? How does it work? And what other Alzheimer's breakthroughs can we expect? Here's everything you need to know:
How does the new drug work?
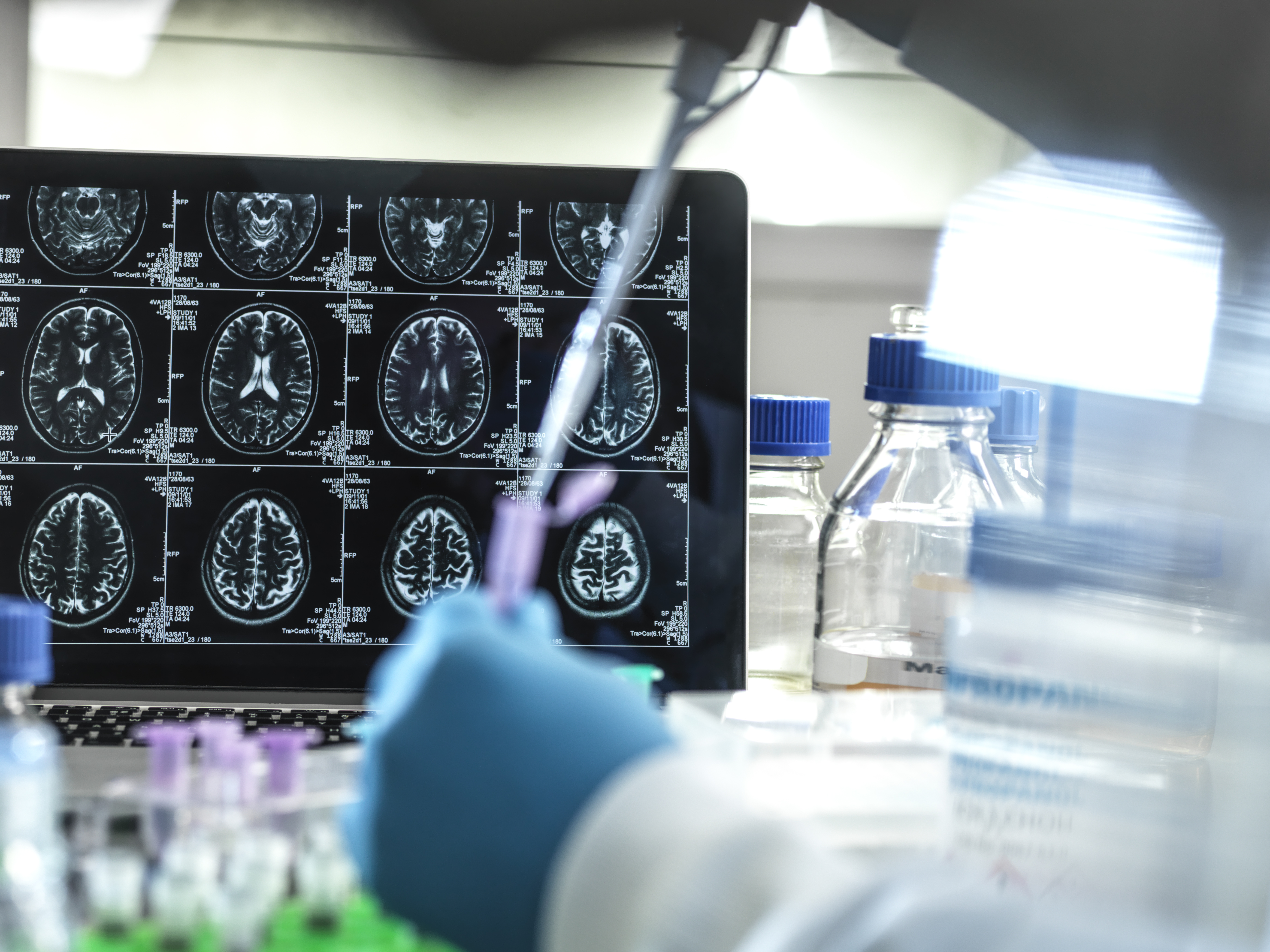
First, you need to understand how Alzheimer's works. Scientists believe that amyloid beta proteins play a role in the disease, according to the National Institute on Aging: "In the Alzheimer's brain, abnormal levels of this naturally occurring protein clump together to form plaques that collect between neurons and disrupt cell function." What lecanemab aims to do, then, is clear out those clumps of protein. According to the trial results, early Alzheimer's patients who took the drug saw significant drops in their amyloid levels after 18 months, while patients who took a placebo saw their amyloid levels rise slightly.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
How effective is it?
Somewhat effective. While the results are a huge breakthrough in Alzheimer's research, some experts caution that the benefits — while real — seem to be modest. Researchers did cognitive testing on patients in both the lecanemab group and the placebo group, and the patients in the drug group showed a moderately slower decline in their mental abilities, the BBC reports. "It's an 18-point scale, ranging from normal through to severe dementia. Those getting the drug were 0.45 points better off." That effect was "modest" one doctor told the news service, "but it gives us a little bit of a foothold."
Are the safety concerns worth it, then?
This might be the biggest question. "It's quite a complicated balancing act for risks and benefits," Rob Howard, a dementia specialist at University College London, tells Nature. The two deaths have raised concerns about the drug's safety, although it should be noted that one of the makers of lecanemab denies culpability in one of the deaths and is still researching the other. (Both patients reportedly had other underlying health issues, and there are suggestions the new drug might not be great for people on blood thinners.) Still, roughly 20 percent of patients who took the drug saw some level of brain swelling or bleeding — but fewer than 3 percent experienced symptoms. "All the available safety information indicates that lecanemab therapy is not associated with an increased risk of death overall," the drug company Eisai said in a statement.
A debate over lecanemab's merits seems likely. "I worry any minor benefit may be washed out by the practical difficulties of living with the drug and the substantial risks associated with taking the drug," Matthew Schrag of Vanderbilt University Medical School, told The Washington Post. But the Alzheimer's Association reacted to the trial results with enthusiasm, saying in a statement the drug "can meaningfully change the course of the disease for people in the earliest stages of Alzheimer's disease."
Aren't there other Alzheimer's drugs out there?
News of lecanemab's success came out the same day the drug company Roche shut down most of the trials for its own Alzheimer's-fighting treatment. The Roche drug was also designed to clear out amyloid blockages, Reuters reports, but "did not show a statistically significant benefit in patients" in the early stages of the disease. Another drug, Aduhelm, was controversially approved for Alzheimer's treatment in 2021; critics say it does little to reverse cognitive decline.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
What's next?
The drug faces an "uncertain" path to federal approval, Axios reports. Lecanemab's makers have submitted an expedited application to the FDA and expect a decision by January, but safety issues "are likely to prompt lively debate on whether the agency should limit who can receive it, or at least impose some precautions."
Meanwhile, more research is expected. While the early results are promising, researchers said, "longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer's disease."
Joel Mathis is a writer with 30 years of newspaper and online journalism experience. His work also regularly appears in National Geographic and The Kansas City Star. His awards include best online commentary at the Online News Association and (twice) at the City and Regional Magazine Association.
