CDC recommends new RSV vaccine for infants under 8 months

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
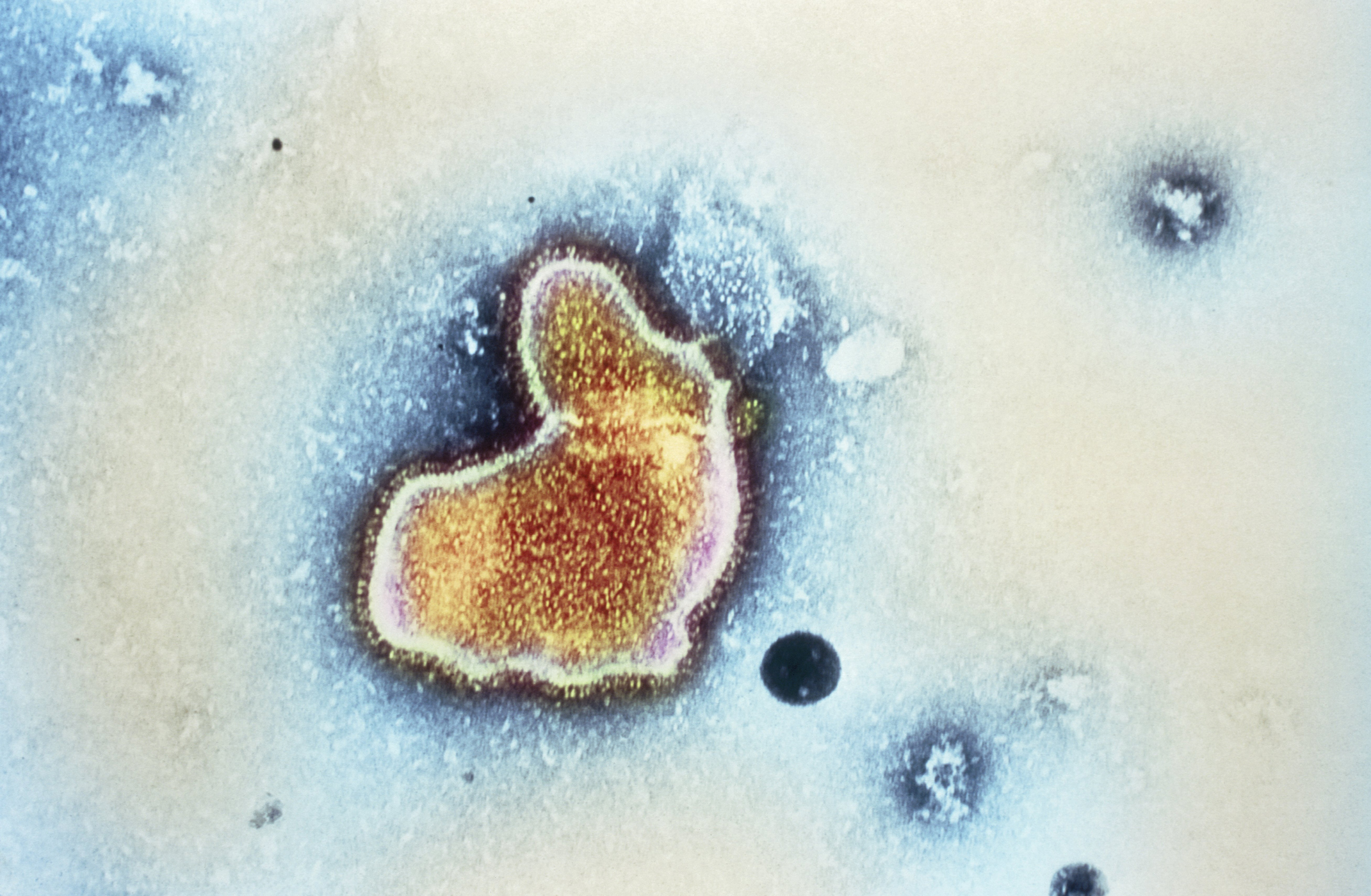
The Centers for Disease Control and Prevention have recommended infants under eight months old get vaccinated for respiratory syncytial virus (RSV) starting in the fall. The disease is currently the "leading cause of hospitalization among infants in the U.S." Yearly, 58,000 to 80,000 children under 5 years of age, most of whom are infants, are hospitalized because of RSV. Some 100 to 300 children die from the disease every year.
The new vaccine, called nirsevimab, was approved in July. It is an antibody vaccine, which varies from typical vaccines. Regular vaccines "train the body's own immune defenses against the virus," while an antibody vaccine "works to directly fend off the virus in the body," CBS News reported. "This new RSV immunization provides parents with a powerful tool to protect their children against the threat of RSV," said CDC director Mandy Cohen. "RSV is the leading cause of hospitalizations for infants and older babies at higher risk and today we have taken an important step to make this life saving product available."
Almost all children will contract RSV by their second birthday, and the risk spikes every year in the winter and early spring months. The virus causes flu-like symptoms including fever, cough and runny nose. In children under three, "the illness may move into the lungs and cause coughing and wheezing," and in some cases "the infection turns to a severe respiratory disease," according to Cedars-Sinai. "I think that we will look back on this, in a short period of time, and see what a major impact this vote has had on the health and wellbeing of children," Dr. José Romero, director of the National Center for Immunization and Respiratory Diseases, said in a meeting for the CDC's Advisory Committee on Immunization Practices, which endorsed giving the vaccine.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Devika Rao has worked as a staff writer at The Week since 2022, covering science, the environment, climate and business. She previously worked as a policy associate for a nonprofit organization advocating for environmental action from a business perspective.
-
 6 exquisite homes with vast acreage
6 exquisite homes with vast acreageFeature Featuring an off-the-grid contemporary home in New Mexico and lakefront farmhouse in Massachusetts
-
 Film reviews: ‘Wuthering Heights,’ ‘Good Luck, Have Fun, Don’t Die,’ and ‘Sirat’
Film reviews: ‘Wuthering Heights,’ ‘Good Luck, Have Fun, Don’t Die,’ and ‘Sirat’Feature An inconvenient love torments a would-be couple, a gonzo time traveler seeks to save humanity from AI, and a father’s desperate search goes deeply sideways
-
 Political cartoons for February 16
Political cartoons for February 16Cartoons Monday’s political cartoons include President's Day, a valentine from the Epstein files, and more
-
 Scientists are worried about amoebas
Scientists are worried about amoebasUnder the radar Small and very mighty
-
 Metal-based compounds may be the future of antibiotics
Metal-based compounds may be the future of antibioticsUnder the radar Robots can help develop them
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Deaths of children under 5 have gone up for the first time this century
Deaths of children under 5 have gone up for the first time this centuryUnder the radar Poor funding is the culprit
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
 More adults are dying before the age of 65
More adults are dying before the age of 65Under the radar The phenomenon is more pronounced in Black and low-income populations
