How close are we to a norovirus vaccine?
A new Moderna trial raises hopes of vanquishing a stomach bug that sickens millions a year


A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Norovirus, often erroneously called the "stomach flu," sickens an estimated 21 million Americans and 685 million people worldwide every year, and though there is currently no preventive vaccine, a promising contender is in the works.
The virus causes several nightmarish days of severe vomiting and diarrhea, often accompanied by fever, body aches and dehydration. The scourge is also maddeningly hardy and highly contagious. Although mortality rates are very low in developed countries, outbreaks can overwhelm emergency rooms, create unwelcome pressure on health care systems and cause widespread absences from work and school. It spreads particularly easily in close quarters, including daycares, cruise ships, universities and nursing homes.
Norovirus is not a form of influenza but rather a calicivirus. New strains appear every two to four years, and are "difficult to wipe out because they can withstand hot and cold temperatures as well as most disinfectants," said The Norovirus Foundation. Norovirus is "more a nauseating nuisance than a public-health crisis," said The Atlantic, but scientists have nevertheless been at work on a norovirus vaccine for years. In September 2024, the first volunteers were dosed in Moderna's advanced clinical trial for a norovirus vaccine that showed encouraging results in preliminary trials.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
What did the commentators say?
Experts have expressed caution about how feasible it will be to deliver a norovirus vaccine. "Will it work here? Keep a bucket handy, just in case," said the American Council on Science and Health in a post on X about the Moderna trial. That's because "norovirus has been devilishly difficult to even contemplate vaccinating against" given how rapidly it mutates, said the BBC. Rolling out an "effective, safe norovirus vaccine has been elusive, primarily because of the virus' high genetic diversity," and its large number of genotypes, said MedPage Today. Some skepticism also stems from the recent failure of a HilleVax vaccine for infants which "failed to meet key endpoints" in its trial in July 2024, said Clinical Trials Arena.
A recent winter surge of norovirus cases in the northern hemisphere, however, has renewed calls for vaccine development. "What better way to remind people of the power of vaccines than to eliminate the misery of puking?" said Alexandra Sifferlin at The New York Times. And while the "norovirus jab won't eliminate the bug, it could significantly reduce its impact on the health system," said The Independent.
What next?
Moderna researchers claim that "earlier trials of the vaccine have shown it generates a strong immune response in humans," and that the new trial is "designed to explore whether the jab is effective against the virus itself and, if so, how long protection lasts," said The Guardian. Moderna's norovirus inoculation uses the same mRNA technology that produced the company's revolutionary Covid-19 vaccines in 2020, which "use a genetic code to tell the body's cells to produce proteins that train the immune system," said Penn Medicine.
Moderna now needs to complete a "phase III trial," which is the final step before drugs or vaccines are submitted for the regulatory approval process in the United States. They are "critical to understanding whether vaccines are safe and effective," said Johns Hopkins University. Most phase III trials are randomized control trials, meaning that "participants and most of the study investigators do not know who has received the vaccine and who received the placebo," and are larger than earlier-phase trials, said Johns Hopkins.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
In the Moderna norovirus trial, volunteers will participate for one year each, which will involve six in-person visits to trial sites as well as some monitoring and follow-up phone calls. The company hopes to enroll 25,000 people from all over the world, which will take two years. That means that even if the vaccine is shown to be safe and effective, it will not be available to the public until late 2026 or 2027. In the meantime, experts recommend washing your hands frequently, "especially after using the restroom, changing diapers and before preparing food or eating" and to avoid cooking food for others while sick, said The Mayo Clinic.
David Faris is a professor of political science at Roosevelt University and the author of "It's Time to Fight Dirty: How Democrats Can Build a Lasting Majority in American Politics." He's a frequent contributor to Newsweek and Slate, and his work has appeared in The Washington Post, The New Republic and The Nation, among others.
-
 Moltbook: The AI-only social network
Moltbook: The AI-only social networkFeature Bots interact on Moltbook like humans use Reddit
-
 Judge orders Washington slavery exhibit restored
Judge orders Washington slavery exhibit restoredSpeed Read The Trump administration took down displays about slavery at the President’s House Site in Philadelphia
-
 Kurt Olsen: Trump’s ‘Stop the Steal’ lawyer playing a major White House role
Kurt Olsen: Trump’s ‘Stop the Steal’ lawyer playing a major White House roleIn the Spotlight Olsen reportedly has access to significant U.S. intelligence
-
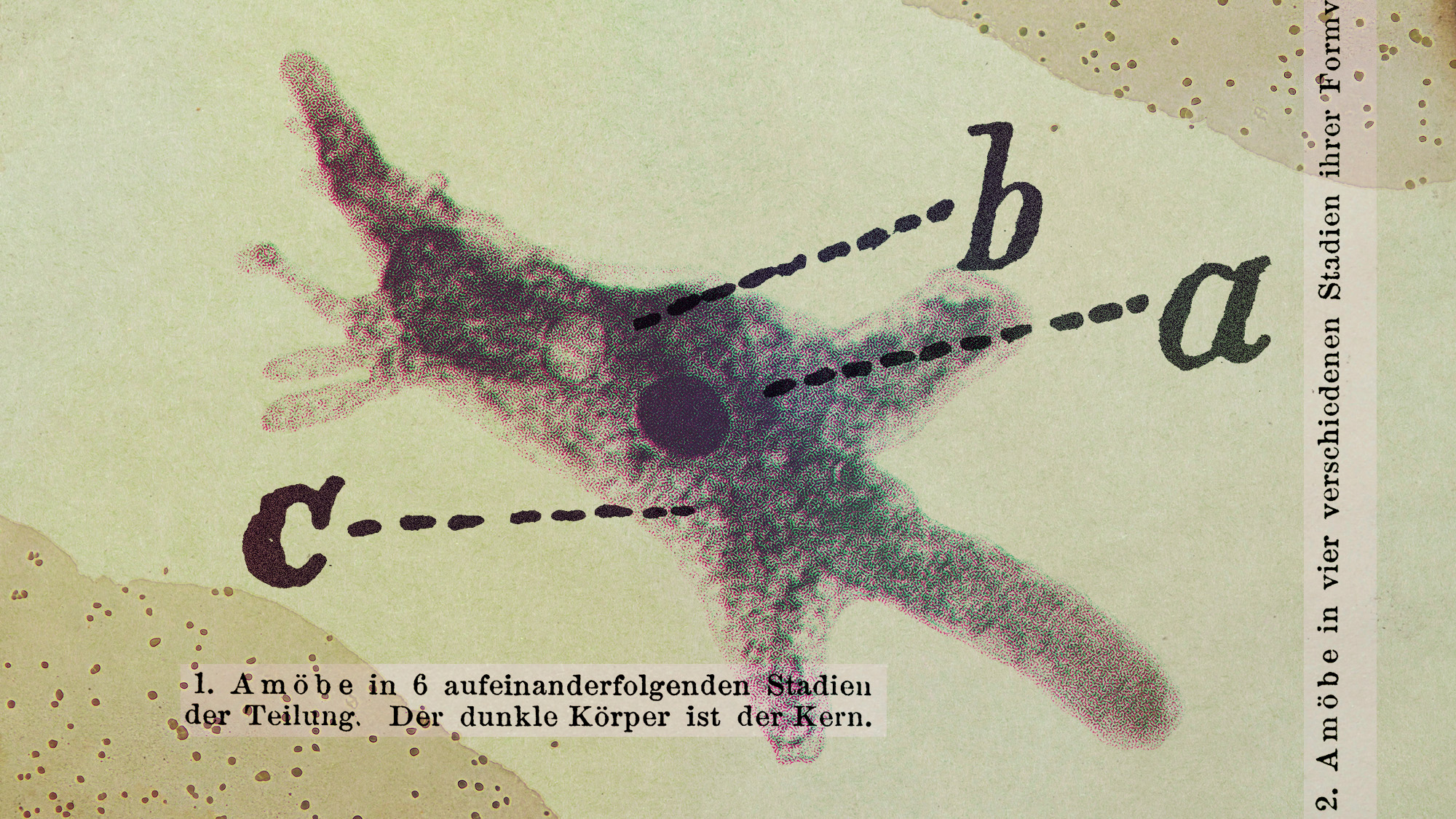 Scientists are worried about amoebas
Scientists are worried about amoebasUnder the radar Small and very mighty
-
 Metal-based compounds may be the future of antibiotics
Metal-based compounds may be the future of antibioticsUnder the radar Robots can help develop them
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 Deaths of children under 5 have gone up for the first time this century
Deaths of children under 5 have gone up for the first time this centuryUnder the radar Poor funding is the culprit
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
 How dangerous is the ‘K’ strain super-flu?
How dangerous is the ‘K’ strain super-flu?The Explainer Surge in cases of new variant H3N2 flu in UK and around the world
