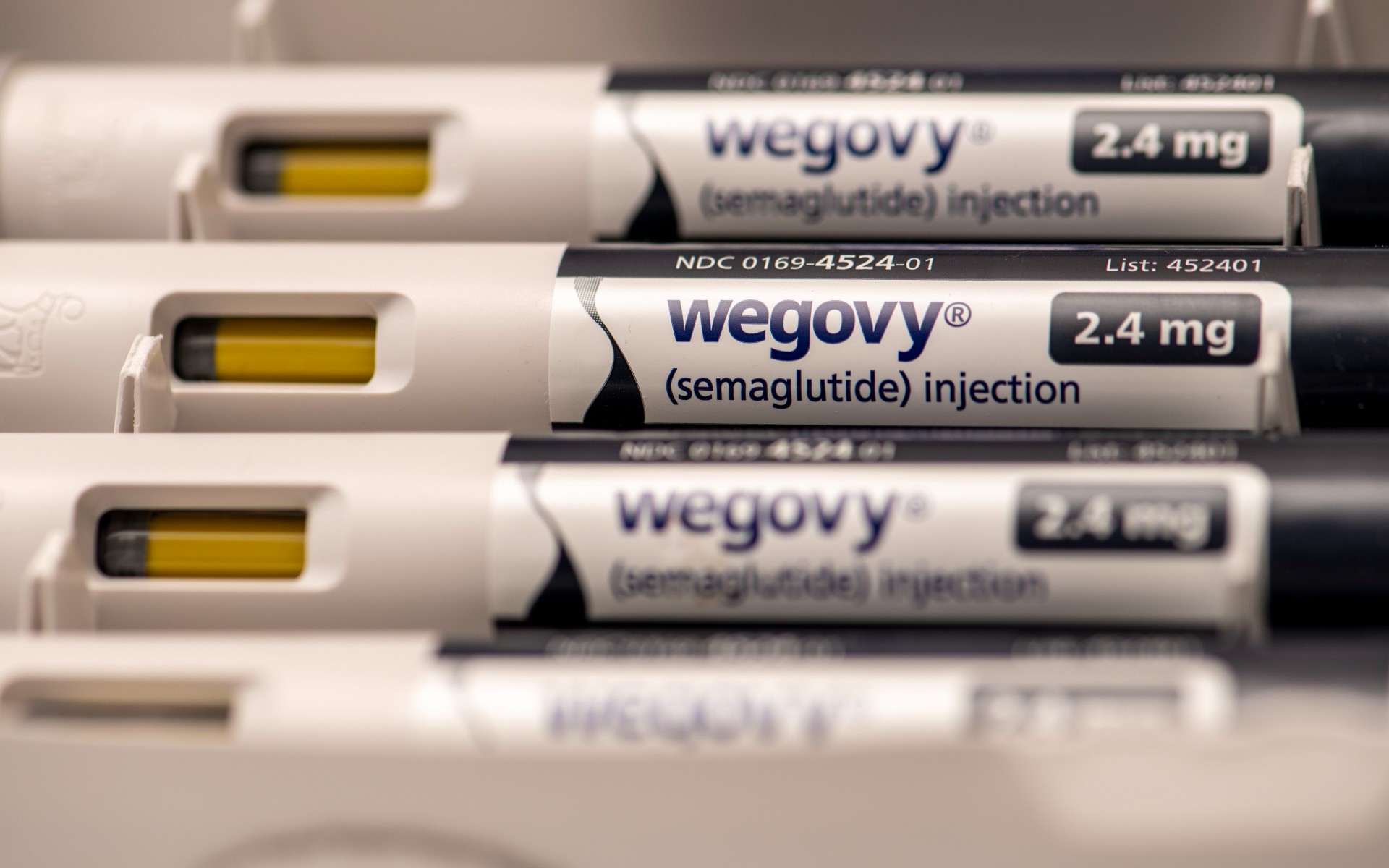
Popular weight loss drugs linked to higher risk of serious gastrointestinal problems
Researchers found that semaglutide, the active ingredient in Wegovy and Ozempic, had a higher risk for side effects like stomach paralysis.

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
Medicines in a popular new class of weight loss drugs including Ozempic and WeGovy may put patients at a higher risk for severe digestive problems, such as stomach paralysis, pancreatitis and bowel obstructions, according to a new study in JAMA Medical Journal. The researchers determined that the side effects were rare but still carried a higher risk than older classes of weight loss medications.
The researchers found that these side effects are rare among individual patients. For example, only about 1% of people taking Ozempic are diagnosed with stomach paralysis, but with demand for the weight loss injectables on the rise, that percentage isn't inconsequential. The study's authors warned that "even rare risks like these may amount to hundreds of thousands of new cases," CNN reported.
"When you have millions of people using these drugs, you know, a 1% risk still translates to many people who may experience these events," said Dr. Mahyar Etminan, an epidemiologist at the University of British Columbia and the lead author of the study. Still, the "study has limits" since it can't prove the drugs caused the side effects, CNN's Brenda Goodman noted.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The study included drugs in the class known as GLP-1 inhibitors. This includes semaglutide, the active ingredient in Wegovy and Novo Nordisk's Ozempic, and liraglutide, the active ingredient in the company's older weight loss medicine Saxenda and diabetes drug Victoza, per Reuters. The researchers compared the drugs to bupropion-naltrexone, the active ingredient in weight-loss drug Contrave, which was approved in 2014.
A Novo Nordisk spokesperson noted that some of the gastrointestinal side effects in the study were already listed on the labels for its weight loss and diabetes medications. The spokesperson added that the company "stands behind the safety and efficacy of all of our GLP-1 medicines when used consistent with the product labeling and approved indications."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Theara Coleman has worked as a staff writer at The Week since September 2022. She frequently writes about technology, education, literature and general news. She was previously a contributing writer and assistant editor at Honeysuckle Magazine, where she covered racial politics and cannabis industry news.
