Pfizer says its vaccine booster provides protection against the Omicron variant

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
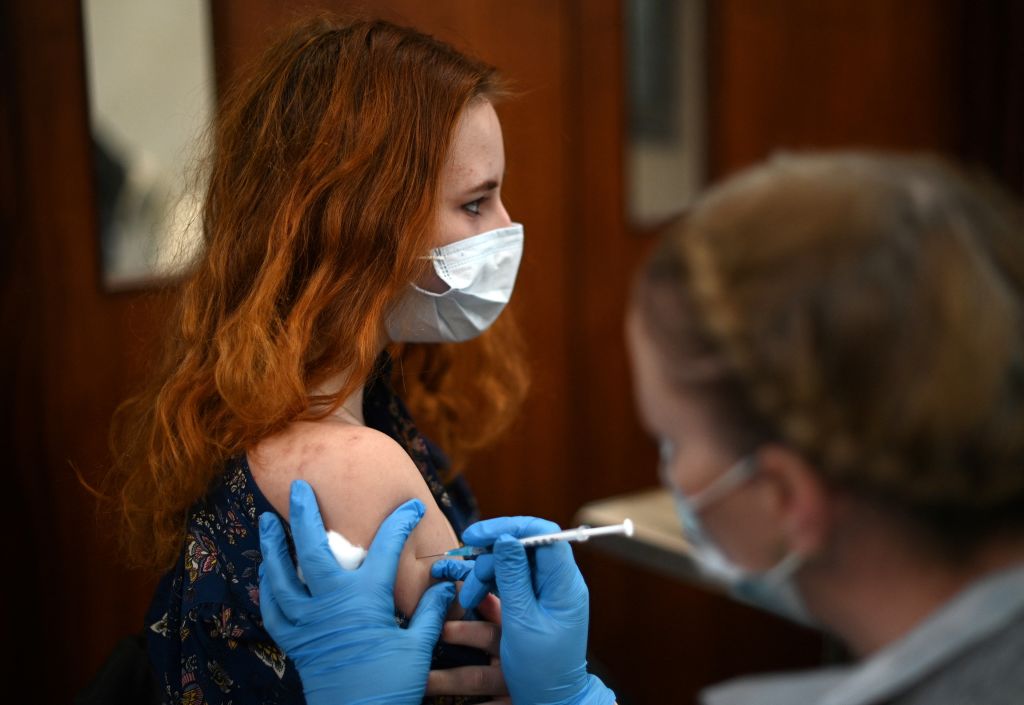
Early data suggests three doses of Pfizer and BioNTech's COVID-19 vaccine provides protection against the new Omicron variant, the companies say.
Pfizer and BioNTech announced Wednesday that a third dose of its COVID-19 vaccine offers a similar amount of protection against the new Omicron variant as two doses of the vaccine does against the original strain of the coronavirus, NBC News reports. The companies also said, however, that preliminary studies indicate two doses "may not be sufficient to protect against infection with the Omicron variant," and a third dose provides "more robust" protection.
"Although two doses of the vaccine may still offer protection against severe disease caused by the Omicron strain, it's clear from these preliminary data that protection is improved with a third dose of our vaccine," Pfizer CEO Albert Bourla said. "Ensuring as many people as possible are fully vaccinated with the first two dose series and a booster remains the best course of action to prevent the spread of COVID-19."
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
BioNTech CEO Ugur Sahin added that "our preliminary, first dataset indicate that a third dose could still offer a sufficient level of protection from disease of any severity caused by the Omicron variant."
The news comes less than two weeks after the World Health Organization designated the new Omicron variant a variant of concern. Asked on the Today show whether this new data suggests a two-shot Pfizer regime is "effectively useless" against Omicron, Bourla stressed that there still is "tremendous value" in receiving two doses "compared to if you only have one or if you don't have any."
Bourla added, "It might not be enough on itself, but we are waiting to see. So you may need to go to get the third booster faster, and that's something that the health authorities should consider very carefully and make their recommendations."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brendan worked as a culture writer at The Week from 2018 to 2023, covering the entertainment industry, including film reviews, television recaps, awards season, the box office, major movie franchises and Hollywood gossip. He has written about film and television for outlets including Bloody Disgusting, Showbiz Cheat Sheet, Heavy and The Celebrity Cafe.
-
 How the FCC’s ‘equal time’ rule works
How the FCC’s ‘equal time’ rule worksIn the Spotlight The law is at the heart of the Colbert-CBS conflict
-
 What is the endgame in the DHS shutdown?
What is the endgame in the DHS shutdown?Today’s Big Question Democrats want to rein in ICE’s immigration crackdown
-
 ‘Poor time management isn’t just an inconvenience’
‘Poor time management isn’t just an inconvenience’Instant Opinion Opinion, comment and editorials of the day
-
 A Nipah virus outbreak in India has brought back Covid-era surveillance
A Nipah virus outbreak in India has brought back Covid-era surveillanceUnder the radar The disease can spread through animals and humans
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 A fentanyl vaccine may be on the horizon
A fentanyl vaccine may be on the horizonUnder the radar Taking a serious jab at the opioid epidemic
-
 Health: Will Kennedy dismantle U.S. immunization policy?
Health: Will Kennedy dismantle U.S. immunization policy?Feature ‘America’s vaccine playbook is being rewritten by people who don’t believe in them’
-
 How dangerous is the ‘K’ strain super-flu?
How dangerous is the ‘K’ strain super-flu?The Explainer Surge in cases of new variant H3N2 flu in UK and around the world
-
 Vaccine critic quietly named CDC’s No. 2 official
Vaccine critic quietly named CDC’s No. 2 officialSpeed Read Dr. Ralph Abraham joins another prominent vaccine critic, HHS Secretary Robert F. Kennedy Jr.
-
 This flu season could be worse than usual
This flu season could be worse than usualIn the spotlight A new subvariant is infecting several countries
-
 Covid-19 mRNA vaccines could help fight cancer
Covid-19 mRNA vaccines could help fight cancerUnder the radar They boost the immune system
