FDA approves startup's portable coronavirus test kit

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
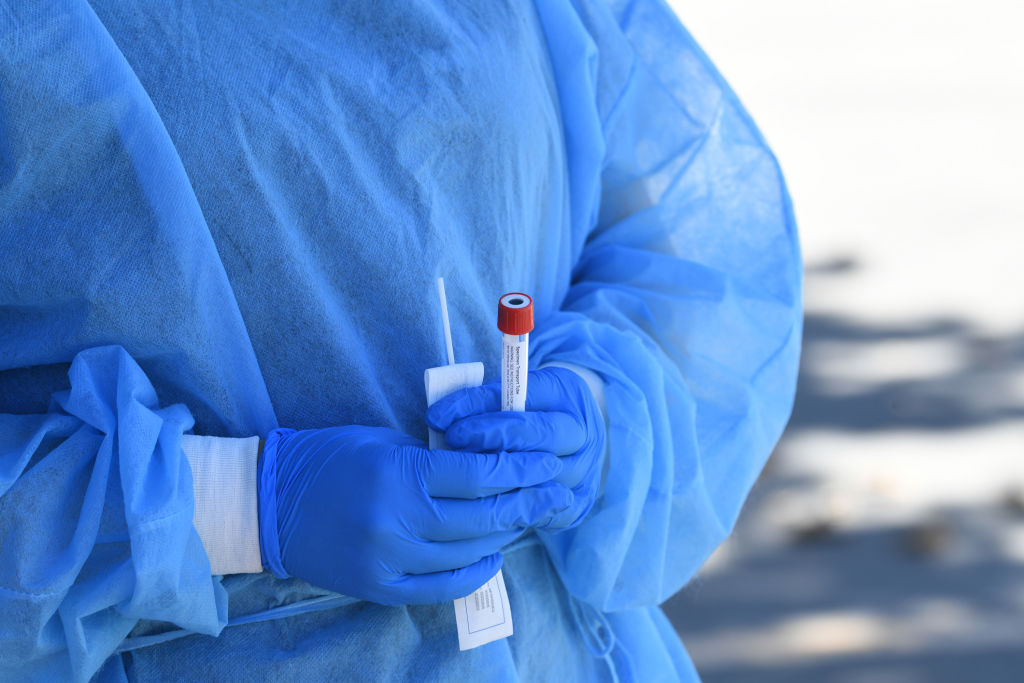
Visby Medical, a startup in Silicon Valley, last week became the first company to receive an Emergency Use Authorization from the Food and Drug Administration for a portable PCR coronavirus test kit, Reuters reports.
PCR testing, considered one of the most accurate diagnostic tools for infectious diseases, usually requires a large, expensive machine to run results, but Visby's product can reportedly fit in a person's palm. The medical equipment company's founder and CEO Adam de la Zerda said he hasn't settled on a price for the kit — which Visby has been developing for the last seven years, initially for the purpose of diagnosing sexually transmitted diseases — but the goal is to make it affordable.
For now, the EUA will allow Visby's kit to be used in clinical labs, where it can process a nasal swab and detect COVID-19 in 30 minutes, but eventually Visby aims to sell it directly to consumers for in-home use, per Reuters.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Steven Chu, who has won the Nobel Prize for physics and invested in Visby, said he believes portable coronavirus test kits "can really transform things." The United States, of course, hopes to increase its testing capacity through affordable, efficient methods from any location, and Visby's kit seems to fit the bill. Read more at Reuters.
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Tim is a staff writer at The Week and has contributed to Bedford and Bowery and The New York Transatlantic. He is a graduate of Occidental College and NYU's journalism school. Tim enjoys writing about baseball, Europe, and extinct megafauna. He lives in New York City.
-
 Political cartoons for February 11
Political cartoons for February 11Cartoons Wednesday's political cartoons include erasing Epstein, the national debt, and disease on demand
-
 The Week contest: Lubricant larceny
The Week contest: Lubricant larcenyPuzzles and Quizzes
-
 Can the UK take any more rain?
Can the UK take any more rain?Today’s Big Question An Atlantic jet stream is ‘stuck’ over British skies, leading to ‘biblical’ downpours and more than 40 consecutive days of rain in some areas
-
 ‘One Battle After Another’ wins Critics Choice honors
‘One Battle After Another’ wins Critics Choice honorsSpeed Read Paul Thomas Anderson’s latest film, which stars Leonardo DiCaprio, won best picture at the 31st Critics Choice Awards
-
 Son arrested over killing of Rob and Michele Reiner
Son arrested over killing of Rob and Michele ReinerSpeed Read Nick, the 32-year-old son of Hollywood director Rob Reiner, has been booked for the murder of his parents
-
 Rob Reiner, wife dead in ‘apparent homicide’
Rob Reiner, wife dead in ‘apparent homicide’speed read The Reiners, found in their Los Angeles home, ‘had injuries consistent with being stabbed’
-
 Hungary’s Krasznahorkai wins Nobel for literature
Hungary’s Krasznahorkai wins Nobel for literatureSpeed Read László Krasznahorkai is the author of acclaimed novels like ‘The Melancholy of Resistance’ and ‘Satantango’
-
 Primatologist Jane Goodall dies at 91
Primatologist Jane Goodall dies at 91Speed Read She rose to fame following her groundbreaking field research with chimpanzees
-
 Florida erases rainbow crosswalk at Pulse nightclub
Florida erases rainbow crosswalk at Pulse nightclubSpeed Read The colorful crosswalk was outside the former LGBTQ nightclub where 49 people were killed in a 2016 shooting
-
 Trump says Smithsonian too focused on slavery's ills
Trump says Smithsonian too focused on slavery's illsSpeed Read The president would prefer the museum to highlight 'success,' 'brightness' and 'the future'
-
 Trump to host Kennedy Honors for Kiss, Stallone
Trump to host Kennedy Honors for Kiss, StalloneSpeed Read Actor Sylvester Stallone and the glam-rock band Kiss were among those named as this year's inductees
