Officials may recommend Johnson & Johnson vaccine resume use 'as soon as this weekend'

A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
You are now subscribed
Your newsletter sign-up was successful
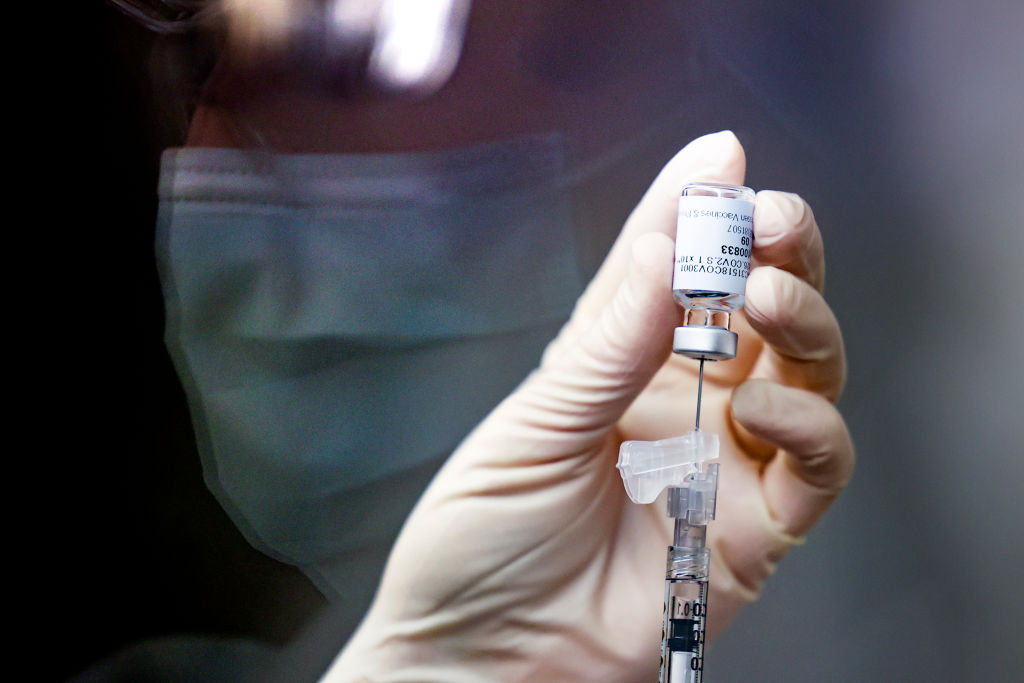
The pause of Johnson & Johnson's COVID-19 vaccine in the United States might be days away from coming to an end, according to a new report.
Federal health authorities are "leaning toward" recommending resuming use of Johnson & Johnson's COVID-19 vaccine, "possibly as soon as this weekend," The Washington Post reported on Thursday.
The Food and Drug Administration and the Centers for Disease Control and Prevention on April 13 called for a pause of the vaccine "out of an abundance of caution" due to "six reported U.S. cases of a rare and severe type of blood clot in individuals after receiving" the vaccine.
The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
On Friday, a meeting of a CDC advisory group is scheduled to take place, and the panel could recommend the vaccine be put back in use. According to the Post, officials will likely not recommend age restrictions for the vaccine but may recommend it come with a warning. That would be a similar step to the one taken by the European Medicines Agency, which said that "unusual blood clots" should be "listed as very rare side effects" of the vaccine, but determined that its benefits outweigh its risks.
CDC Director Rochelle Walensky while speaking to NBC's Today on Thursday said that she didn't want to "get ahead" of the advisory committee, but is "really hopeful that we'll be able to use the vaccine soon." Walensky also noted to the Post that the government has only seen a "handful" of additional blood clotting cases and that "we are not being inundated with things that we are concerned about," while Acting FDA Commissioner Janet Woodcock said the lack of a "huge avalanche" of clotting cases is a "great relief."
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brendan worked as a culture writer at The Week from 2018 to 2023, covering the entertainment industry, including film reviews, television recaps, awards season, the box office, major movie franchises and Hollywood gossip. He has written about film and television for outlets including Bloody Disgusting, Showbiz Cheat Sheet, Heavy and The Celebrity Cafe.
-
 Magazine solutions - February 13, 2026
Magazine solutions - February 13, 2026Puzzle and Quizzes Magazine solutions - February 13, 2026
-
 The pros and cons of tapping your 401(k) for a down payment
The pros and cons of tapping your 401(k) for a down paymentpros and cons Does it make good financial sense to raid your retirement for a home purchase?
-
 Music reviews: Ari Lennox, Lucinda Williams, and A$AP Rocky
Music reviews: Ari Lennox, Lucinda Williams, and A$AP RockyFeature ‘Vacancy,’ ‘World’s Gone Wrong,’ and ‘Don’t Be Dumb’
-
 Trump HHS slashes advised child vaccinations
Trump HHS slashes advised child vaccinationsSpeed Read In a widely condemned move, the CDC will now recommend that children get vaccinated against 11 communicable diseases, not 17
-
 FDA OKs generic abortion pill, riling the right
FDA OKs generic abortion pill, riling the rightSpeed Read The drug in question is a generic version of mifepristone, used to carry out two-thirds of US abortions
-
 RFK Jr. vaccine panel advises restricting MMRV shot
RFK Jr. vaccine panel advises restricting MMRV shotSpeed Read The committee voted to restrict access to a childhood vaccine against chickenpox
-
 Texas declares end to measles outbreak
Texas declares end to measles outbreakSpeed Read The vaccine-preventable disease is still spreading in neighboring states, Mexico and Canada
-
 RFK Jr. shuts down mRNA vaccine funding at agency
RFK Jr. shuts down mRNA vaccine funding at agencySpeed Read The decision canceled or modified 22 projects, primarily for work on vaccines and therapeutics for respiratory viruses
-
 Measles cases surge to 33-year high
Measles cases surge to 33-year highSpeed Read The infection was declared eliminated from the US in 2000 but has seen a resurgence amid vaccine hesitancy
-
 Kennedy's vaccine panel signals skepticism, change
Kennedy's vaccine panel signals skepticism, changeSpeed Read RFK Jr.'s new vaccine advisory board intends to make changes to the decades-old US immunization system
-
 Kennedy ousts entire CDC vaccine advisory panel
Kennedy ousts entire CDC vaccine advisory panelspeed read Health Secretary RFK Jr. is a longtime anti-vaccine activist who has criticized the panel of experts
